Salvage surgery after high-dose radiotherapy
Introduction
In 1895, X-rays were discovered by a German physicist, Wilhelm Conrad Rontgen. Soon thereafter their diagnostic but also their therapeutic potential was discovered. Ever since that time, they have been indispensable in the diagnosis and treatment of cancer. At the present time, surgery and radiotherapy are the most important local treatment modalities for lung cancer (1). Specific radiation delivery is an ever changing domain in the therapeutic armamentarium for lung cancer and recently, specific techniques have been developed allowing high-dose delivery to tumor cells with sparing of critical organs in close contact to the primary tumor (1).
At the present day, some subgroups of early-stage non-small cell lung cancer (NSCLC), especially those with a compromised cardiopulmonary function, may be treated by stereotactic radiotherapy (SRT), also called stereotactic body radiation therapy (SBRT), stereotactic ablative radiotherapy (SABR), or stereotactic radiosurgery (SRS). The latter term is confusing and should be avoided as surgical treatment is not a part of this specific therapy. For the sake of simplicity in this manuscript we prefer to use the term SRT which is currently considered to be an alternative to surgical treatment.
Treatment of locally advanced NSCLC remains highly controversial, mainly due to the fact that this represents a very heterogeneous population ranging from unexpected nodal involvement to bulky mediastinal lymph node metastases which do not qualify for surgical resection (2). The main treatment options comprise definitive concurrent chemoradiation which has become the standard treatment, sequential chemoradiation, induction chemotherapy followed by surgery or radiotherapy, and induction concurrent chemoradiation followed by surgery.
For those patients having persistent or recurrent disease after high-dose SRT or after full chemoradiation, salvage surgery has emerged as a possible treatment option (3). Salvage surgery is defined as surgical resection of persistent or recurrent primary lung cancer after previous local treatment without surgery, in case of urgency such as hemoptysis, bronchial stenosis, abscess cavity or empyema, or as therapy for chronic bronchopleural or bronchopulmonary fistulas (Table 1). It is considered to be technically more difficult due to post-radiation inflammation and fibrosis.
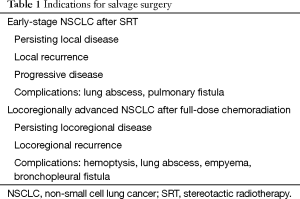
Full table
Every patient qualifying for salvage surgery should undergo a thorough cardiopulmonary evaluation and be discussed within a dedicated multidisciplinary tumor board, especially when a pneumonectomy or intrapericardial dissection is anticipated. Up till now, only limited data have been published. A best evidence topic on salvage surgical interventions after high-dose radiotherapy was published by Schreiner and colleagues in 2015 (4). In total, 4 out of 9 reports provided the best available evidence but all of them were retrospective. In 47 patients, 48 salvage resections were performed after high-dose radiotherapy, SRT or chemoradiation. There were 4 sublobar resections, 32 (bi)lobectomies (1 sleeve resection), and 12 pneumonectomies. Mean postoperative complication rate extracted from 4 studies was 42.5%. Median survival time ranged from 9 till 30 months. The authors concluded that salvage surgery may be considered a worthwhile treatment option with low mortality, acceptable morbidity and relatively good survival.
In the present review, salvage surgery after high-dose radiotherapy is discussed, making a clear distinction between surgical resection after SRT for early-stage lung cancer, and surgery after combined modality therapy for locoregionally advanced disease.
Salvage surgery in early-stage lung cancer
Approximately 20% of patients diagnosed with NSCLC have early-stage lung cancer. In the recently introduced 8th TNM edition further subdivisions are made in the T (tumor) descriptor with increments of 1 cm for T1 and T2 lung cancer, as prognosis is best for the smallest lung cancers (5). According to the guidelines of the European Society of Medical Oncology (ESMO) lobectomy is still considered the standard surgical treatment for early-stage lung cancer (6). In patients who are unable to undergo a lobectomy, but fit for surgery, sublobar resections represent an alternative option and are preferred over radiotherapy (7,8). In total, 10% to 15% of the patients, diagnosed with early-stage NSCLC are medically unfit for surgery or decline surgery and these patients are usually treated with SRT (9). An ultra-high dose is administered consisting of ablative fractions of radiation to a target, which allows for maximizing cell-killing effect on the primary tumor (1). In contrast to conventional irradiation, which is delivered daily for six to eight weeks, SRT is typically administered in one to ten fractions in doses of 6–34 Gy per fraction (10). Good results have been reported with SRT in medically inoperable patients with a local control rate of 80–100% and 3-year survival of 40–80% (10,11). However, it should be noted that a histological diagnosis is not obtained in every case, lymph node evaluation and clinical staging are less rigorous, and no universally accepted criteria are available for response evaluation (12). In this way, local control is often defined as “absence of progressive disease” in contrast to a complete surgical resection which implies that there is no remaining disease (13-15).
The promising results of SRT in medically unfit patients stimulated further research of SRT in patients with a good cardiopulmonary function as alternative treatment to surgical resection. Onishi et al. investigated SRT in stage I and II patients who were medically fit but refused surgery (16). SRT (45–72.5 Gy in 3–10 fractions) was associated with a 5-year cumulative local control rate of 92% for T1 tumors and 73% for T2 tumors. Five-year overall survival (OS) rates were 72% for stage IA and 62% for stage IB, respectively, similar to outcomes reported in surgical series (16). The U.S. STARS (NCT 00840749) and Dutch ROSEL (NCT00687986) trials were randomized controlled trials comparing surgical resection to SRT but closed early due to poor accrual. Data were pooled in a subsequent analysis by Chang et al. showing a significant 3-year OS advantage in favor of SRT (95% vs. 79%; P=0.037) in the 58 included patients (17). Disease-free survival was not different between both groups. The authors state that these data suggest at least clinical equipoise between the two treatment modalities. Several Letters to the Editor pointed out that results cannot be generalized as mortality in the surgical arm was unacceptably high, detailed histology was not obtained in every case and a valid comparison of locoregional control between surgery and SRT is not possible (14,18-23). Further randomised studies are currently ongoing mainly recruiting patients at higher risk for surgical resection (24).
A recent question that emerged and which is currently discussed at major lung cancer conferences is how to treat locoregional recurrences after SRT and whether surgery may play a role as subsequent treatment. As mentioned before, specific diagnosis of persisting or recurrent tumor growth after SRT is challenging as an inflammatory reaction is induced by SRT giving rise to pneumonitis and fibrosis at the level of the primary tumor site. The appearance of a zone of consolidation on chest computed tomography (CT) could be radiation pneumonitis, radiation fibrosis, persisting tumor growth or local recurrence (25). Tumor serum markers as carcinoembryonic antigen (CEA) and fluorodeoxyglucose positron-emission tomographic (FDG-PET) images may be helpful in the differential diagnosis although increased FDG-uptake may persist up to 2 years after SRT without evidence of recurrence (26,27).
Reported local recurrence rate after SRT for early-stage NSCLC mounts to 20% after 3 years (10). A larger tumor size and a lower retention index on FDG-PET scanning are risk factors for recurrence (28,29). Time between SRT and local recurrence is highly variable, ranging from 9 to 89 months (30). Therefore, close follow-up after SRT is indispensable.
Treatment options for local recurrence after SRT include salvage surgery, systemic chemotherapy, targeted agents, chemoradiation, repeat high-dose radiotherapy or best supportive therapy in patients with poor performance status (30-32). Indications for salvage surgery are listed in Table 1 (Figures 1,2). Reassessment of cardiopulmonary functional operability at the time of local recurrence is very important. Rather surprisingly, patients undergoing salvage surgery may have been considered inoperable at the time of SRT as was the case in the patient described in Figures 1,2, and in 3 out of 12 patients in the series reported by Hamaji and colleagues (30). On the other hand, operable patients may become functionally inoperable over time giving rise to a high operative risk, and in these cases alternative options have to be considered (32).
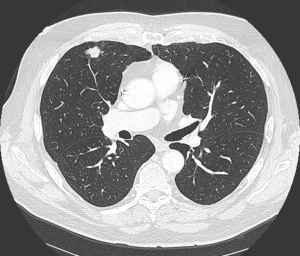
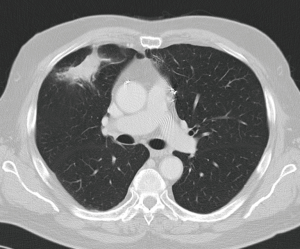
Regarding literature data only retrospective series are available. Hamaji et al. reported a series of 49 patients with isolated local recurrence after SRT (30). Best supportive care was administered in 29 patients, chemoradiation in 8, and 12 patients underwent salvage surgery. For the latter subgroup 5-year OS was 79.5% calculated from local recurrence on.
Sugimoto et al. reported a right upper lobectomy by video-assisted thoracic surgery (VATS) for a peripheral stage I NSCLC after radiotherapy (33). Adhesions were limited and easily divided. Central structures were intact, probably because of peripheral and not central irradiation. Neri and colleagues reported 7 patients who underwent salvage surgery after SRT for primary NSCLC or lung metastases (26). Median time period between SRT and salvage surgery was 10 months. There were 6 lobectomies and 1 segmentectomy; 2 patients had incomplete resections. The distance from the pleura to the tumor ranged from 5 to 30 mm. There were no technical difficulties and complications consisted of 1 pulmonary fistula and 4 cases of post-radiotherapy pneumonitis. Allibhai et al. reported a small but interesting series as detailed pathological examination was available (34). Out of a prospective series of 209 patients who underwent SRT for early-stage NSCLC 4 had salvage surgery consisting of lobectomy and nodal sampling. In one patient a partial chest wall resection was necessary. Pleural adhesions were encountered in every case but there were no significant technical complications. No viable tumor was found in one patient but the others had 5–65% viable cancer cells. After a median follow-up of 30 months there was no evidence of disease. Although in the cases of Hamaji, Sugimoto and Neri, only limited pleural adhesions were present after SRT, more extensive adhesions were encountered by Allibhai and also in 2 out of 9 patients published by Verstegen, which led to conversion in one case (34,35). This may be due to a different location of the primary lesion and therefore a low radiation dose to the ipsilateral bronchial tree (33,35).
In a cumulative review presented at the 2016 Annual Meeting of the American Association for Thoracic Surgery (AATS) in Baltimore, 37 patients from 4 institutions underwent salvage surgery for pulmonary lesions previously treated with SRT (36). Twenty-six patients had NSCLC and 11 lung metastases with a median disease-free survival of 19.2 months. Of interest, 8 patients (21.6%) were initially considered medically inoperable. Median time interval between SRT and surgery was 16.1 months (range, 6.4–104.0 months). Median survival time following surgery was 46.9 months and 3-year OS 70.1% (36).
In selected cases of oligometastatic disease salvage surgery may also be considered after discussion within a dedicated multidisciplinary team (37). Recently, we reported a case of salvage lung surgery in a patient with oligometastatic disease who initially presented with a femoral bone metastasis from NSCLC treated by osteosynthesis and local radiotherapy (38). Chemoradiation was administered to the primary lung cancer located in the right upper lobe. Because of isolated local recurrence in the upper lobe, salvage lobectomy was performed 18 months later. After present follow-up of 8 years there are no signs of recurrent disease. This case indicates that salvage surgery may even be indicated in exceptional and complex cases on the condition that a complete resection is obtained.
Salvage surgery in locoregionally advanced lung cancer
At the time of lung cancer diagnosis 30% of the patients present with locoregionally advanced disease comprising stages IIIA and IIIB which include quite heterogeneous patient populations (2,39). For this reason treatment of stage IIIA and IIIB NSCLC remains controversial and should be tailored to the individual patient after discussion within a multidisciplinary tumor board. As many patients have extensive mediastinal invasion, concurrent chemoradiation with a high radiotherapy dose (>59 Gy) has become standard treatment in this patient population (1). For potentially operable patients with N2 involvement or T3–4 superior sulcus tumors, induction chemoradiation consists of a locally applied dose of 30–50 Gy in combination with systemic cisplatin-based chemotherapy followed by surgery 4 to 8 weeks later when there is no progressive disease.
Isolated local relapse occurs in 24–35% of patients after definitive chemoradiation for locally advanced NSCLC (40). There is no consensus on the most effective therapy of those recurrences and treatment options include repeat irradiation, systemic chemotherapy, chemoradiation, cryotherapy, radiofrequency ablation, watchful waiting and salvage surgery (41-43). In selected patients salvage surgery may improve survival if a complete resection can be obtained. Specific indications are listed in Table 1. General data in this patient category are more limited but without any doubt, surgical procedures are technically more challenging compared to interventions after SRT for early-stage lung cancer (Figures 3,4). There are multiple reasons for this difference. By definition, locally advanced tumors are more centrally located which provokes hilar and mediastinal fibrosis after high-dose radiotherapy. In its turn, this fibrotic reaction renders dissection of the pulmonary artery more hazardous and often, intrapericardial control of the pulmonary vessels is required. If complications as a lung abscess or an infected cavity occur, the technical complexity of the intervention is further increased with a higher incidence of postoperative complications as empyema and bronchopleural fistula with their associated high mortality rate (44).
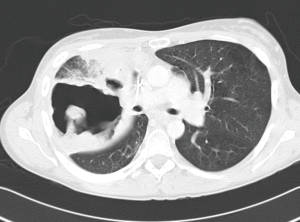
Compared to induction chemoradiation the applied radiation dose in chemoradiation with curative intent is higher (>59 Gy) and the interval between radiation and intervention for recurrence is usually longer than two months. Following this period the tissue response to radiation is more pronounced as demonstrated in a rat model showing that the early post-radiation phase (6 to 12 weeks) is characterized by parenchymal and vascular inflammation, whereas in the late phase (34 to 38 weeks) fibroblast hypercellularity and collagen deposition are the main features (45). Increasing the interval between radiation and operation may give rise to a more difficult identification, manipulation and dissection of tissue, therefore leading to a more tedious intervention resulting in increased blood loss compared to a standard procedure. Major procedures may be necessary with clamping of intrapericardial pulmonary vessels or chest wall resection which prolong operative time (46). Subsequently, a more protracted postoperative course may be anticipated with a higher incidence of postoperative morbidity.
Already in 2008, Bauman and colleagues reported a retrospective series of 24 consecutive patients who underwent salvage resection after definitive high-dose radiation >59 Gy for NSCLC, 22 having concurrent chemotherapy (47). In total, 25 resections were performed, mostly (bi)lobectomies and pneumonectomies. One patient died of adult respiratory distress syndrome and 14 patients experienced perioperative morbidity, 8 of them having at least one major complication. No bronchopleural fistulas were recorded probably due to extensive protection of the stump which in 19 cases was covered by a vascularized flap consisting of 16 omental flaps, 2 intercostal muscle pedicles and 1 pericardial fat pad (47). Viable tumor was present in 19 patients. Median OS was 30 months and estimated 3-year survival rate 47%.
Schreiner and colleagues recently described 9 patients who underwent salvage surgery after definitive chemoradiation for a locally advanced NSCLC with a median radiation dose of 66.2 Gy (48). Median interval between chemoradiation and salvage surgery was 30.2 weeks. There was one postoperative death. Median OS time was 23 months, median progression-free survival 21 months, and overall 3-year survival rate 47%, similar to the results of Bauman et al. (47).
Shimada et al. reported a series of 18 patients, either with relapse or persistent tumor after a median radiotherapy dose of 60 Gy, who underwent salvage surgery (49). In 13 patients, a salvage lobectomy was performed and in 5 patients, a pneumonectomy was performed. There were no operative deaths but complications occurred in 28% of patients. Complete resection was obtained in 89% with a complete pathological response in 28%. Three-year overall and recurrence-free survival rates were 78% and 72%, respectively (49).
These reports show that salvage surgery after full-dose chemoradiation for locally advanced disease is feasible but technically more complex compared to salvage resection after SRT for peripheral, early-stage lung cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- McCloskey P, Balduyck B, Van Schil PE, et al. Radical treatment of non-small cell lung cancer during the last 5 years. Eur J Cancer 2013;49:1555-64. [Crossref] [PubMed]
- Eberhardt WE, De Ruysscher D, Weder W, et al. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol 2015;26:1573-88. [Crossref] [PubMed]
- Uramoto H. Current Topics on Salvage Thoracic Surgery in Patients with Primary Lung Cancer. Ann Thorac Cardiovasc Surg 2016;22:65-8. [Crossref] [PubMed]
- Schreiner W, Dudek W, Sirbu H. Is salvage surgery for recurrent non-small-cell lung cancer after definitive non-operative therapy associated with reasonable survival? Interact Cardiovasc Thorac Surg 2015;21:682-4. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Vansteenkiste J, Crino L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [Crossref] [PubMed]
- De Waele M, Van Schil P. Limited resections in high-risk patients. Curr Opin Pulm Med 2015;21:309-13. [Crossref] [PubMed]
- Sihoe AD, Van Schil P. Non-small cell lung cancer: when to offer sublobar resection. Lung Cancer 2014;86:115-20. [Crossref] [PubMed]
- Robinson CG, Bradley JD. The treatment of early-stage disease. Semin Radiat Oncol 2010;20:178-85. [Crossref] [PubMed]
- Simone CB 2nd, Dorsey JF. Additional data in the debate on stage I non-small cell lung cancer: surgery versus stereotactic ablative radiotherapy. Ann Transl Med 2015;3:172. [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010;28:5153-9. [Crossref] [PubMed]
- Van Schil PE. Salvage surgery after stereotactic radiotherapy: a new challenge for thoracic surgeons. J Thorac Oncol 2010;5:1881-2. [Crossref] [PubMed]
- Van Schil PE, Van Meerbeeck J. Surgery or radiotherapy for early-stage lung cancer--a potential comparison bias. Lancet Oncol 2013;14:e390. [Crossref] [PubMed]
- Van Schil PE. Results of surgery for lung cancer compared with radiotherapy: do we speak the same language. J Thorac Oncol 2013;8:129-30. [Crossref] [PubMed]
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Meyers BF, Puri V, Broderick SR, et al. Lobectomy versus stereotactic body radiotherapy for stage I non-small cell lung cancer: Post hoc analysis dressed up as level-1 evidence? J Thorac Cardiovasc Surg 2015;150:468-71. [Crossref] [PubMed]
- Cao C, D'Amico T, Demmy T, et al. Surgery versus SABR for resectable non-small-cell lung cancer. Lancet Oncol 2015;16:e370-1. [Crossref] [PubMed]
- Zhang L, Tian J, Wang C. Surgery versus SABR for resectable non-small-cell lung cancer. Lancet Oncol 2015;16:e371-2. [Crossref] [PubMed]
- Chang JY, Senan S, Smit EF, et al. Surgery versus SABR for resectable non-small-cell lung cancer - Authors' reply. Lancet Oncol 2015;16:e374-5. [Crossref] [PubMed]
- Dearman C, van As N, Crellin A, et al. Surgery versus SABR for resectable non-small-cell lung cancer. Lancet Oncol 2015;16:e373-4. [Crossref] [PubMed]
- Opitz I, Rocco G, Brunelli A, et al. Surgery versus SABR for resectable non-small-cell lung cancer. Lancet Oncol 2015;16:e372-3. [Crossref] [PubMed]
- McDonald F, De Waele M, Hendriks LE, et al. Management of stage I and II nonsmall cell lung cancer. Eur Respir J 2017;49. [Crossref] [PubMed]
- Takeda T, Takeda A, Kunieda E, et al. Radiation injury after hypofractionated stereotactic radiotherapy for peripheral small lung tumors: serial changes on CT. AJR Am J Roentgenol 2004;182:1123-8. [Crossref] [PubMed]
- Neri S, Takahashi Y, Terashi T, et al. Surgical treatment of local recurrence after stereotactic body radiotherapy for primary and metastatic lung cancers. J Thorac Oncol 2010;5:2003-7. [Crossref] [PubMed]
- Hoopes DJ, Tann M, Fletcher JW, et al. FDG-PET and stereotactic body radiotherapy (SBRT) for stage I non-small-cell lung cancer. Lung Cancer 2007;56:229-34. [Crossref] [PubMed]
- Dunlap NE, Larner JM, Read PW, et al. Size matters: a comparison of T1 and T2 peripheral non-small-cell lung cancers treated with stereotactic body radiation therapy (SBRT). J Thorac Cardiovasc Surg 2010;140:583-9. [Crossref] [PubMed]
- Satoh Y, Nambu A, Onishi H, et al. Value of dual time point F-18 FDG-PET/CT imaging for the evaluation of prognosis and risk factors for recurrence in patients with stage I non-small cell lung cancer treated with stereotactic body radiation therapy. Eur J Radiol 2012;81:3530-4. [Crossref] [PubMed]
- Hamaji M, Chen F, Matsuo Y, et al. Treatment and Prognosis of Isolated Local Relapse after Stereotactic Body Radiotherapy for Clinical Stage I Non-Small-Cell Lung Cancer: Importance of Salvage Surgery. J Thorac Oncol 2015;10:1616-24. [Crossref] [PubMed]
- Verstegen NE, Lagerwaard FJ, Hashemi SM, et al. Patterns of Disease Recurrence after SABR for Early Stage Non-Small-Cell Lung Cancer: Optimizing Follow-Up Schedules for Salvage Therapy. J Thorac Oncol 2015;10:1195-200. [Crossref] [PubMed]
- Hearn JW, Videtic GM, Djemil T, et al. Salvage stereotactic body radiation therapy (SBRT) for local failure after primary lung SBRT. Int J Radiat Oncol Biol Phys 2014;90:402-6. [Crossref] [PubMed]
- Sugimoto S, Toyooka S, Suzawa K, et al. Thoracoscopic lobectomy as salvage surgery for local recurrence of non-small cell lung cancer after carbon ion radiotherapy in an initially operable patient. Ann Thorac Cardiovasc Surg 2014;20 Suppl:501-4. [Crossref] [PubMed]
- Allibhai Z, Cho BC, Taremi M, et al. Surgical salvage following stereotactic body radiotherapy for early-stage NSCLC. Eur Respir J 2012;39:1039-42. [Crossref] [PubMed]
- Verstegen NE, Maat AP, Lagerwaard FJ, et al. Salvage surgery for local failures after stereotactic ablative radiotherapy for early stage non-small cell lung cancer. Radiat Oncol 2016;11:131. [Crossref] [PubMed]
- Antonoff MB, Correa A, Sepesi B, et al. Salvage pulmonary resection following SBRT: a feasible and safe option for local failure. AATS Annual Meeting 2016. Baltimore, 2016.
- Van Schil PE, Hendriks JM, Carp L, et al. Surgery for oligometastatic disease in non-small-cell lung cancer. Expert Rev Anticancer Ther 2008;8:1931-8. [Crossref] [PubMed]
- Duchateau N, Van Bouwel E, Van Schil PE. Salvage surgery in case of oligometastatic disease: a case report. ATS_30276. Ann Thorac Surg 2017. In Press.
- Van Schil PE, De Waele M, Hendriks JM, et al. Surgical treatment of stage III non-small cell lung cancer. Eur J Cancer 2009;45 Suppl 1:106-12. [Crossref] [PubMed]
- Jeppesen SS, Schytte T, Jensen HR, et al. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: an updated retrospective study on local failure and survival rates. Acta Oncol 2013;52:1552-8. [Crossref] [PubMed]
- Bradley J. New territory: surgical salvage for stereotactic body radiation therapy failures in lung cancer. J Thorac Oncol 2010;5:1879-80. [Crossref] [PubMed]
- Goto T, Izumi Y, Nakatsuka S, et al. Percutaneous cryoablation as a salvage therapy for local recurrence of lung cancer. Ann Thorac Surg 2012;94:e31-3. [Crossref] [PubMed]
- Schoellnast H, Deodhar A, Hsu M, et al. Recurrent non-small cell lung cancer: evaluation of CT-guided radiofrequency ablation as salvage therapy. Acta Radiol 2012;53:893-9. [Crossref] [PubMed]
- Van Schil PE, Hendriks JM, Lauwers P. Focus on treatment complications and optimal management surgery. Transl Lung Cancer Res 2014;3:181-6. [PubMed]
- Novakova-Jiresova A, van Luijk P, van Goor H, et al. Changes in expression of injury after irradiation of increasing volumes in rat lung. Int J Radiat Oncol Biol Phys 2007;67:1510-8. [Crossref] [PubMed]
- Samson P, Keogan K, Crabtree T, et al. Interpreting survival data from clinical trials of surgery versus stereotactic body radiation therapy in operable Stage I non-small cell lung cancer patients. Lung Cancer 2017;103:6-10. [Crossref] [PubMed]
- Bauman JE, Mulligan MS, Martins RG, et al. Salvage lung resection after definitive radiation (>59 Gy) for non-small cell lung cancer: surgical and oncologic outcomes. Ann Thorac Surg 2008;86:1632-8; discussion 1638-9. [Crossref] [PubMed]
- Schreiner W, Dudek W, Lettmaier S, et al. Should salvage surgery be considered for local recurrence after definitive chemoradiation in locally advanced non-small cell lung cancer? J Cardiothorac Surg 2016;11:9. [Crossref] [PubMed]
- Shimada Y, Suzuki K, Okada M, et al. Feasibility and efficacy of salvage lung resection after definitive chemoradiation therapy for Stage III non-small-cell lung cancer. Interact Cardiovasc Thorac Surg 2016;23:895-901. [Crossref] [PubMed]