Whack-a-mole strategy for multifocal ground glass opacities of the lung
General principles on ground glass opacity (GGO)
GGO is a good prognostic indicator for lung cancer and is useful for physicians to predict prognosis (1-4). Due to recent advances in computed tomography (CT) and refinement of CT, the chances to encounter GGO are rapidly increasing in clinical practice. Studies on radiological pathological correlation have shown that GGO represents pathological lepidic growth and consolidation on CT represents pathological invasive components. Central scar in adenocarcinoma of the lung has been considered to be the origin of the lung cancer (5), but this “scar cancer” concept was corrected by Shimosato et al. (6). Shimosato et al. insisted that the scar was not the origin but rather representative of pathological invasiveness. Based on that concept Noguchi et al. classified adenocarcinoma of the lung into six grades according to prognosis (7). The presence of active fibroblast in the scar indicated invasiveness and the size of scar itself was also prognostic (8). Thus, a small scar represents small consolidation on thin-section CT, and consolidation tumor ratio 0.5 or less indicates pathological less invasiveness for lung cancer (1,9). Some GGOs have scattered consolidation that is difficult to measure in size (10-12), and GGO dominant tumors show excellent prognosis. Pulmonary lobectomy remains the mainstay treatment for early staged resectable lung cancer based on the findings of randomized trials conducted by the Lung Cancer Study Group in 1995 (13). However, quite a few studies have shown that sublobar resection is equivalent to lobectomy for radiological early lung cancers (14-16). Indication of sublobar resection remains unclear as it results in lethal locoregional recurrence especially after wedge resection and that is related to an insufficient surgical margin (17,18). We have conducted a phase III trial of sublobar resection for radiologically non-invasive lung cancers, JCOG0804, and will present the results in the near future. As such, GGO dominant tumors could be cured by local treatment alone such as sublobar resection when an adequate surgical margin was present.
Management of GGO
One of the most important missions for physicians who encounter a newly detected GGO lesion on thin-section CT is to perform an observation. The observation discriminates transient GGO from persistent GGO. Transient GGO is one that disappears during follow up (19,20). The optimal follow-up time is two or three months. Approximately 40–50% of GGOs have been reported to disappear. If a GGO remains during the follow-up, the lesion is called a persistent GGO. Management of the persistent GGO should be observation or surgical intervention. Radiation exposure with CT during follow up could be one of the disadvantages of observation (21), and this problem would be emphasized in young patients.
Classification of GGO
Typical classification of GGOs based on the findings of thin-section CT is as follows: Pure GGO, part-solid GGO, and solid (22). The classification, however, is confusing, as a GGO with consolidation tumor ratio 0.25 is dealt as pure GGO rather than part-solid GGO (2,4,15,23-28) by some. There might be a GGO with a scattered solid part and the solid part of the tumor cannot be evaluated using conventional methods (10,11). The author has attempted to classify those GGOs into six categories (Table 1): pure GGO, pure GGO with a denser appearance, GGO predominant part-solid, GGO with scattered consolidation, solid predominant part-solid tumor, and solid. This is the tentative classification of GGO used in clinical practice at our institute (10-12,29).
Natural history of GGO
GGOs of the lung intact in size are mostly lung adenocarcinomas or atypical adenomatous hyperplasia (30). As mentioned above, we have completed a non-randomized confirmatory phase III study of sublobar surgical resection for peripheral GGO dominant lung cancers defined with thoracic thin-section CT (JCOG0804/WJOG4507L). This study was conducted to analyze relapse free survival (RFS) in patients who underwent sublobar resection for peripherally located radiological early lung cancer. Mode of surgery was basically wedge resection and segmentectomy was allowed when surgical margin was insufficient (<5 mm) or histological invasiveness was present. Radiological GGO dominant lung cancer was defined as lung cancer 2.0 cm or less in maximum tumor dimension and having consolidation tumor ratio 0.25 or less in diameter. All of these lesions were “persistent GGO” and median consolidation tumor ratio was 0 indicating that most tumors were pure GGOs. Histological diagnosis was adenocarcinomas in 90%, and precancerous lesion in 5% (30). As for clinical behavior of such GGO lesions, some stay or increase in size, and others increase in density on thin-section CT. Such a natural history of subsolid nodule (SSN) should be considered when selecting treatment for the lesions. Based on the study in which 1,238 SSNs were followed for a median of 4.3 years, approximately 20% and 50% of pure GGOs and part-solid tumors, respectively, increase in size in five years (23). Predictors of increasing in size were male, age (60 years or elder), maximum tumor dimension 10 mm or more, smoking and history of lung cancer for pure GGOs. Several papers have dealt with stepwise progression from the GGO stage towards invasive adenocarcinoma (31,32). Size of some GGOs remains unchanged for several years and then starts to increase (33). These reports support the fact that follow-up of GGOs should continue indefinitely.
Optimal surgical intervention for GGOs
The lung cancer study group conducted one of the most important clinical trials on the optimal mode of surgery for early stage T1 lung cancer (13). This study concluded that sublobar resection would result in three times more locoregional recurrence and poor prognosis compared with pulmonary lobectomy. Basically, pulmonary lobectomy is indicated for small sized lung cancers where occult nodal metastasis cannot be ignored, and the same is true even for subcentimeter lung cancers (34). On the other hand, sublobar resection would be sufficient for GGO dominant tumors, which are minimally invasive lung cancers pathologically (16,35-38). With regard to mode of surgery, wide wedge resection is less invasive than segmentectomy (1) and wide wedge resection is preferred for compromised patients. However local recurrence after wedge resection is much higher than segmentectomy and the indication of wide wedge resection should be decided with caution (13). Even for radiologically early lung cancers, wedge resection would result in lethal local recurrences (17,18,26). Cut-end recurrence was found in 5 (19%) of 26 patients who underwent wedge resection for GGO dominant tumor (17). The surgical margin should be confirmed as adequate whenever wedge resection for lung cancer is performed, and this is a lesson learnt from the high percentage of local recurrences. Thus segmentectomy is better for lung cancer oncologically, but is not as good as wedge from the stand point of surgical invasiveness. Segmental resection for lung cancer was reported to be equivalent to lobectomy (16,35-37) and this has been confirmed by at least two randomized trials conducted in Japan and US (39). Controversies still remains as to the optimal mode of surgery for lung cancer which shows a solid appearance on thin-section CT scan for high locoregional recurrence has been reported after sublobar resection (40).
Management of multiple lung nodules
Multiple lung nodules were recently divided into four categories (41). These are second primary lung cancer, separate tumor nodules, multifocal GGOs, and the diffuse pneumonic type (Figure 1). Each TNM staging is needed for double primary lung cancers. TNM staging is needed for the primary tumor for separate lung nodules and multifocal GGOs. Double primary lung cancers should be treated based on the stage of more advanced tumors. Separate lung nodules should be divided into two categories based on nodal status and CT appearance of satellite nodules, intrapulmonary metastasis or multiple primary lung cancers. Multifocal primary lung cancers belonging to separate lung nodules or multifocal GGOs have a dominant lung cancer and separate nodules that have GGO appearance on thin-section CT (Figure 2). Such invasive lung cancers with multifocal GGOs show less invasive pathological findings compared with solitary invasive lung cancers (42). The treatment strategy for those tumors should be considered based on the stage of the dominant lesion. The indication of surgical intervention for GGO is as follows: Pure GGO 15 mm or more in maximum tumor dimension on lung view of thin-section CT, denser GGO, ground glass predominant part-solid with CTR more than 0.25, GGO with scattered solid, and solid predominant part-solid (10-12,29,43). If an additional tumor is located on the ipsilateral side, simultaneous resection of the remaining GGO would be considered. For the remaining tumors located on the contralateral side, staged operation would be indicated. Some GGO lesions could be candidates for observation only, and the risk of radiation exposure should be taken into consideration during follow up especially for patients under 50 years old (21).

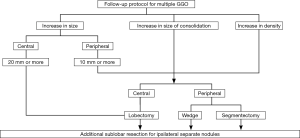
Follow-up protocol for multifocal GGO—“Whack-a-mole strategy”
The goal of the follow up of unresected GGO lesions is to avoid step-wise progression to invasive lung cancer which may result in metastasis (31,32). According to the follow-up data, approximately 30% of patients have multiple lung lesions (23). During the following up of GGOs, some retain their status, and others change in size or density on thin-section CT (23,44-48). As mentioned above, the pathological invasive part correlates with the solid part on thin-section CT, and enlarged solid part should be more serious than enlarged maximum tumor dimension of the tumor (1,8). Surgical intervention should be considered for GGNs where the solid part increased in size or density became higher (Figure 3). Peripherally located GGOs are easy to excise and the indication for surgical intervention is easier to decide. Which sublobar resection is appropriate should be decided based on the surgical margin during surgery in order to prevent lethal cut-end recurrence (17,18). An enlarged pure GGO during follow up is not so invasive pathologically compared with an enlarged solid part. That means more waiting until surgical intervention can be allowed. For elder patients in particular, there is no need to adopt surgery indefinitely. Deciding upon surgical intervention for centrally located GGO is challenging. Surgery for centrally located tumors would be pulmonary lobectomy in most cases except for bilateral superior segments of the lower lobe. Based on our above strategy we have treated 131 patients with multiple lung cancers of clinical N0 status, and 53 patients had multifocal GGO lesions (43). Median for multifocal GGOs ranging from 2 to 41 in number was 3. Five-year survival for patients with multiple GGOs does not differ between the modes of surgery, namely, sublobar resection and lobectomy. Further investigation is warranted, however our tentative strategy is considered to be feasible.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological non-invasiveness in peripheral clinical IA lung cancer (JCOG0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Suzuki K, Asamura H, Kusumoto M, et al. "Early" peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg 2002;74:1635-9. [Crossref] [PubMed]
- Takamochi K, Nagai K, Yoshida J, et al. Pathologic N0 status in pulmonary adenocarcinoma is predictable by combining serum carcinoembryonic antigen level and computed tomographic findings. J Thorac Cardiovasc Surg 2001;122:325-30. [Crossref] [PubMed]
- Aoki T, Tomoda Y, Watanabe H, et al. Peripheral lung adenocarcinoma: correlation of thin-section CT findings with histologic prognostic factors and survival. Radiology 2001;220:803-9. [Crossref] [PubMed]
- Yokoo H, Suckow EE. Peripheral lung cancers arising in scars. Cancer 1961;14:1205-15. [Crossref] [PubMed]
- Shimosato Y, Suzuki A, Hashimoto T, et al. Prognostic implications of fibrotic focus (scar) in small peripheral lung cancers. Am J Surg Pathol 1980;4:365-73. [Crossref] [PubMed]
- Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844-52. [Crossref] [PubMed]
- Suzuki K, Yokose T, Yoshida J, et al. Prognostic significance of the size of central fibrosis in peripheral adenocarcinoma of the lung. Ann Thorac Surg 2000;69:893-7. [Crossref] [PubMed]
- Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. [Crossref] [PubMed]
- Ichikawa T, Hattori A, Suzuki K, et al. Clinicopathological characteristics of lung cancer mimicking organizing pneumonia on computed tomography-a novel radiological entity of pulmonary malignancy. Jpn J Clin Oncol 2016;46:681-6. [Crossref] [PubMed]
- Matsunaga T, Suzuki K, Hattori A, et al. Lung cancer with scattered consolidation: detection of new independent radiological category of peripheral lung cancer on thin-section computed tomography. Interact Cardiovasc Thorac Surg 2013;16:445-9. [Crossref] [PubMed]
- Suzuki K, Kusumoto M, Watanabe S, et al. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg 2006;81:413-9. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non- small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Watanabe S, Watanabe T, Arai K, et al. Results of wedge resection for focal bronchioloalveolar carcinoma showing pure ground-glass attenuation on computed tomography. Ann Thorac Surg 2002;73:1071-5. [Crossref] [PubMed]
- Nakata M, Saeki H, Takata I, et al. Focal ground-glass opacity detected by low-dose helical CT. Chest 2002;121:1464-7. [Crossref] [PubMed]
- Yamato Y, Tsuchida M, Watanabe T, et al. Early results of a prospective study of limited resection for bronchioloalveolar adenocarcinoma of the lung. Ann Thorac Surg 2001;71:971-4. [Crossref] [PubMed]
- Nakao M, Yoshida J, Goto K, et al. Long-term outcomes of 50 cases of limited-resection trial for pulmonary ground-glass opacity nodules. J Thorac Oncol 2012;7:1563-6. [Crossref] [PubMed]
- Yoshida J, Ishii G, Yokose T, et al. Possible delayed cut-end recurrence after limited resection for ground-glass opacity adenocarcinoma, intraoperatively diagnosed as Noguchi type B, in three patients. J Thorac Oncol 2010;5:546-50. [Crossref] [PubMed]
- Felix L, Serra-Tosio G, Lantuejoul S, et al. CT characteristics of resolving ground-glass opacities in a lung cancer screening programme. Eur J Radiol 2011;77:410-6. [Crossref] [PubMed]
- Oh JY, Kwon SY, Yoon HI, et al. Clinical significance of a solitary ground-glass opacity (GGO) lesion of the lung detected by chest CT. Lung Cancer 2007;55:67-73. [Crossref] [PubMed]
- Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ 2017;356:j347. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Kakinuma R, Noguchi M, Ashizawa K, et al. Natural History of Pulmonary Subsolid Nodules: A Prospective Multicenter Study. J Thorac Oncol 2016;11:1012-28. [Crossref] [PubMed]
- Park JH, Lee KS, Kim JH, et al. Malignant pure pulmonary ground-glass opacity nodules: prognostic implications. Korean J Radiol 2009;10:12-20. [Crossref] [PubMed]
- Kim HK, Choi YS, Kim K, et al. Management of ground-glass opacity lesions detected in patients with otherwise operable non-small cell lung cancer. J Thorac Oncol 2009;4:1242-6. [Crossref] [PubMed]
- Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005;129:991-6. [Crossref] [PubMed]
- Nakata M, Sawada S, Saeki H, et al. Prospective study of thoracoscopic limited resection for ground-glass opacity selected by computed tomography. Ann Thorac Surg 2003;75:1601-5; discussion 1605-6. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Yokouchi H, et al. Natural history of pure ground-glass opacity after long-term follow-up of more than 2 years. Ann Thorac Surg 2002;73:386-92; discussion 392-3. [Crossref] [PubMed]
- Matsunaga T, Suzuki K, Takamochi K, et al. What is the radiological definition of part-solid tumour in lung cancer?†. Eur J Cardiothorac Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Suzuki K, Watanabe S, Mizusawa J, et al. Predictors of non-neoplastic lesions in lung tumours showing ground-glass opacity on thin-section computed tomography based on a multi-institutional prospective study†. Interact Cardiovasc Thorac Surg 2015;21:218-23. [Crossref] [PubMed]
- Min JH, Lee HY, Lee KS, et al. Stepwise evolution from a focal pure pulmonary ground-glass opacity nodule into an invasive lung adenocarcinoma: an observation for more than 10 years. Lung Cancer 2010;69:123-6. [Crossref] [PubMed]
- Soda H, Nakamura Y, Nakatomi K, et al. Stepwise progression from ground-glass opacity towards invasive adenocarcinoma: long-term follow-up of radiological findings. Lung Cancer 2008;60:298-301. [Crossref] [PubMed]
- Takahashi S, Tanaka N, Okimoto T, et al. Long term follow-up for small pure ground-glass nodules: implications of determining an optimum follow-up period and high-resolution CT findings to predict the growth of nodules. Jpn J Radiol 2012;30:206-17. [Crossref] [PubMed]
- Hattori A, Suzuki K, Matsunaga T, et al. Prognostic Significance of the Standardized Uptake Value on Positron Emission Tomography in Patients with Multiple Clinical-N0 Lung Cancers. Thorac Cardiovasc Surg 2015;63:597-603. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg 2003;125:924-8. [Crossref] [PubMed]
- Tsubota N, Ayabe K, Doi O, et al. Ongoing prospective study of segmentectomy for small lung tumors. Study Group of Extended Segmentectomy for Small Lung Tumor. Ann Thorac Surg 1998;66:1787-90. [Crossref] [PubMed]
- Kodama K, Doi O, Higashiyama M, et al. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg 1997;114:347-53. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Locoregional recurrence after segmentectomy for clinical-T1aN0M0 radiologically solid non-small-cell lung carcinoma†. Eur J Cardiothorac Surg 2017;51:518-25. [PubMed]
- Detterbeck FC, Bolejack V, Arenberg DA, et al. The IASLC Lung Cancer Staging Project: Background Data and Proposals for the Classification of Lung Cancer with Separate Tumor Nodules in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:681-92.
- Hattori A, Matsunaga T, Takamochi K, et al. Oncological Characteristics of Radiological Invasive Adenocarcinoma with Additional Ground-Glass Nodules on Initial Thin-Section Computed Tomography: Comparison with Solitary Invasive Adenocarcinoma. J Thorac Oncol 2016;11:729-36. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Surgical Management of Multifocal Ground-Glass Opacities of the Lung: Correlation of Clinicopathologic and Radiologic Findings. Thorac Cardiovasc Surg 2017;65:142-9. [PubMed]
- Aoki T, Hanamiya M, Uramoto H, et al. Adenocarcinomas with predominant ground-glass opacity: correlation of morphology and molecular biomarkers. Radiology 2012;264:590-6. [Crossref] [PubMed]
- Yoshida Y, Kokubu A, Suzuki K, et al. Molecular markers and changes of computed tomography appearance in lung adenocarcinoma with ground-glass opacity. Jpn J Clin Oncol 2007;37:907-12. [Crossref] [PubMed]
- Attinà D, Niro F, Stellino M, et al. Evolution of the subsolid pulmonary nodule: a retrospective study in patients with different neoplastic diseases in a nonscreening clinical context. Radiol Med 2013;118:1269-80. [Crossref] [PubMed]
- Cho S, Yang H, Kim K, et al. Pathology and prognosis of persistent stable pure ground-glass opacity nodules after surgical resection. Ann Thorac Surg 2013;96:1190-5. [Crossref] [PubMed]
- Matsuguma H, Mori K, Nakahara R, et al. Characteristics of subsolid pulmonary nodules showing growth during follow-up with CT scanning. Chest 2013;143:436-43. [Crossref] [PubMed]