Thoracic sympathectomy for hyperhidrosis: from surgical indications to clinical results
Introduction and brief historical remarks
The birth of sympathetic surgery dates from the end of nineteenth century. Initially, little was known about the physiology of the autonomic nervous system and so, surgical indications were imprecise and results, anecdotal. The first sympathectomy in History was described by Alexander (1) in 1889, to treat an epileptic patient. In 1920, the first sympathectomy to treat hyperhidrosis was done by Kotzareff (2). In 1942, Hughes (3) published the first thoracoscopic sympathectomy and with the development of minimally invasive techniques/instruments, open surgical procedures for the thoracic sympathetic chain have become progressively obsolete. In present days, endoscopic thoracic sympathectomy (ETS) is performed by minimally invasive videothoracoscopy, and in our opinion, there are no arguments to justify any open surgery to address the thoracic sympathetic trunk.
Simultaneously to the evolution of the surgical technique during the twentieth century, the knowledge on the physiology of the autonomic nervous system has evolved as well and, as a consequence, the surgical indications have been profoundly modified. Nowadays, the main indication for ETS is palmar primary hyperhidrosis. Undoubtedly, the best results are achieved in these patients. However, the procedure can also be performed with good results in well-selected cases of axillar hyperhidrosis and facial hyperhidrosis/facial blushing.
Much less frequently, ETS can still be indicated and lead to satisfactory outcomes in highly selected patients with some specific cardiologic disorders, and in a few painful and/or ischemic conditions to the upper limbs.
Hyperhidrosis: definitions, pathophysiology, clinical features and diagnosis
Hyperhidrosis can be defined as a pathologic condition characterized by excessive sweating beyond the organism’s physiological needs to maintain the body temperature within an adequate range.
This condition can be didactically divided into primary or secondary. Primary hyperhidrosis is the pathology itself, characterized by the excessive sweating which exceeds the physiological needs for a normal thermoregulation. It occurs in about 2% of the world population, affecting equally men and women, and it seems to have a genetic predisposition (4).
The exact pathophysiology of primary hyperhidrosis is not totally clarified yet. It is already known that the disease is not caused by abnormalities on the eccrine sweat glands themselves, since they are histologically and functionally normal in affected individuals (5). More than just “excessive quantity of sweat”, primary hyperhidrosis seems to be related to a dysfunction on the thermoregulation capacity of the sympathetic component of the autonomic nervous system, in which there is a huge disproportion between the trigger represented by stress and/or ambient heat conditions and the body response represented by sweat production. When triggered, the sweat overproduction promptly begins and keeps on going like a “short-circuit”, regardless the persistence (or not) of the stimulus. Quite often, excessive sweating occurs even without an obvious trigger.
In primary hyperhidrosis, the excessive sweat is usually localized and can be described mostly as palmar (hands), axillar (armpits), and/or plantar (feet). Craniofacial hyperhidrosis can also occur, alone or with facial blushing. Patients with primary hyperhidrosis classically present with sweating complaints dating from the first decade of life (remarkably in hands) and during the adolescence the symptoms normally become more relevant, leading to a negative impact in quality of life (QoL). Physical, psychological and emotional stresses tend to worsen the symptoms, which are not related to the ambient temperature and are clearly disproportional to it. Another important aspect of the primary hyperhidrosis is that sweating episodes occur only when the patient is awake.
In the other hand, secondary hyperhidrosis presents as a consequence secondary to another disorder, which means that hyperhidrosis is not the disease itself, but only a clinical manifestation of another underlying process. The sweating is usually generalized and the onset of symptoms is normally in adulthood, coinciding with the inception of the underlying disease. Episodes can occur when the patient is awake or sleeping and the influence of stress is less evident. Among the possible causes are the following: local heat, obesity, systemic disorders such as hyperthyroidism, diabetes mellitus, pre/peri-menopause disturbances, neuroendocrine tumors, hematologic malignancies, neurologic diseases, spinal cord injuries, as well as tuberculosis and other infections. Secondary hyperhidrosis can also be pharmacologically induced with continued use of certain medications, such as antidepressants. Whatever the etiology, patients with secondary hyperhidrosis should not be considered surgical candidates and must be referred to clinical treatment focused on the underlying cause of the sweating.
A careful clinical evaluation based mainly on patient’s history, complemented by physical examination, are the most valuable tools and are usually enough to establish the diagnosis of primary hyperhidrosis. In the majority of cases, laboratory and radiological tests are not necessary, but may be required according to clinical judgment. Specific exams to objectively quantify sweat production indeed exist (6-9), but they are not routinely used in clinical practice.
Nonsurgical treatment of primary hyperhidrosis
A wide variety of clinical treatments can be tried for primary hyperhidrosis (10). These treatments are indeed less invasive than a surgical intervention, but all of them have only a palliative potential and, once interrupted the therapy, the symptoms will relapse in virtually all patients. From a practical point of view, it means that the majority of patients will eventually end up seeking for a surgical consultation.
Medical treatment is a nonsurgical tool to control hyperhidrosis, but only with palliative intent, and can be associated with other therapeutic strategies. Anticholinergic drugs (oxybutynin, glycopyrrolate, propantheline) can be used, but their long-term use is frequently limited by side effects. Patients with hyperhidrosis/blushing linked to anxiety and emotional triggers can be treated with benzodiazepines and beta-blockers, besides psychological therapy.
Botulinum toxin A (Botox©—Allergan Inc., Irvine, California, USA) local injections are frequently used to control hyperhidrosis, with good results. The main problems related to this treatment are time-limited efficacy and the fact that injections must repeated every three to six months to sustain the effects. The process requires many painful punctures at the sweating site and cost may also become an issue. If injections are not repeated, symptoms reappear. Many patients who come for surgical consultation have already experienced one or more botulinum toxin injections.
Iontophoresis is another conservative therapy. It is based on the application of electrical currents over the affected areas, which must be immersed in an ionized solution. Although the procedure is well tolerated and good results are reported, iontophoresis is not widely used, since its effects are temporary and the technique must be repeated every two to three days in the beginning of treatment and then weekly to maintain its effects.
Sweat glands ablation by energy-based devices is a promising nonsurgical alternative to treat hyperhidrosis, especially for axillary cases. The approved devices by FDA use microwave energy (MyraDry©—Miramar Labs Inc., Sunnyvale, California, USA), high-intensity focused ultrasound (Ulthera System©—Ulthera Incorporated, Mesa, Arizona, USA) or laser therapy. Regardless the type of energy used, the principle is to promote the ablation and destruction of the sweat glands in the affected area (usually armpits). Considering the fact that eccrine sweat glands do not regenerate, these therapies theoretically could be capable to eradicate sweat permanently, what could potentially avoid surgery in many patients with axillary hyperhidrosis.
The use of aluminum-based antiperspirants specifically developed for axillary hyperhidrosis is a common practice with dismal long-term results. There is also a wide diversity of topical solutions, which vary on their active components, but none of them has proved to be long-lasting effective in controlling excessive sweating.
Axillary hyperhidrosis patients can also be treated with local surgeries, focused on the mechanical removal of local tissue containing axillary sweat glands, by resection, liposuction (11) and/or curettage (12). These modalities may avoid an ETS and, as with any other surgical approach, there are potential advantages/benefits and drawbacks/risks involved. Nevertheless, these procedures are not the scope of this paper and will not be addressed in details.
The STS consensus for surgical treatment of Hyperhidrosis
When reading publications about ETS, sometimes is difficult to precise where and how the sympathetic trunk has been interrupted. In these situations, analysis and comparison between techniques and results is difficult or even impossible to be made.
Considering this issue, the Society of Thoracic Surgeons (STS) has published in 2011 a consensus for the surgical treatment of hyperhidrosis (13), after a broad review of the literature. It suggests an unified nomenclature regarding surgical aspects of hyperhidrosis treatment, as well as a standardized handling of the sympathetic trunk for each type of primary hyperhidrosis, defining the exact levels to be approached according to the localization of the sweating complaints of each patient (hands, armpits, feet, and/or face).
Sympathetic surgery for the treatment of hyperhidrosis
Appropriate patient selection for surgery
The optimal postoperative result is highly dependent on the adequate selection of the right patient for the right procedure.
As highlighted before, the patient’s history—based on the sweating complaints, its location, intensity, triggers, age of onset and other information—is the most valuable tool to define the diagnosis of primary hyperhidrosis. Theoretically, patients can be submitted to ETS at any age, provided that the available surgical instruments are adequate for the patient’s intercostal spaces and thoracic cavity’s dimensions (14).
Once the diagnostic is confirmed and the surgical indication is well established, we prefer to propose ETS to patients who have age and discernment enough to precise to what extent hyperhidrosis is actually something that negatively impacts their QoL enough to justify the procedure, with all its potential risks and benefits. In other words, we do not operate children just because their parents ask for it, even if the diagnosis is clear. Respected this principle, it worth to mention the evidence that a younger age might be a predictor of good outcome after sympathectomy, regarding the risk of compensatory hyperhidrosis (CH), personal satisfaction and cure of symptoms (15).
During their initial consultation, patients and their parents also must be fully and clearly informed about the pathology, all treatment options available and the potential benefits, drawbacks, expected results and possible complications of each one of them (including undesired effects, specially the risk of CH). A conscious and well-informed individual tends to be a more motivated patient, and this will likely create more realistic expectations, with a lesser risk of frustrations and regretting after surgery.
The best results after ETS are achieved in patients with palmar hyperhidrosis, in which symptoms disappearance and QoL improvement is near to 100%. In these patients, ETS should be considered as first-line treatment. Those with palmar-axillar complaints are also among the most satisfied after surgery, majorly because of the control of the hand sweating.
In the other hand, patients with isolated axillary hyperhidrosis experiment a lower degree of satisfaction after sympathectomy and should be referred to other treatment modalities before considering an ETS.
Patients with facial hyperhidrosis and/or blushing constitute a particular group: their outcomes after sympathectomy are very effective with respect to cure of symptoms; Nevertheless, due to the need to interrupt the sympathetic trunk in a high level (second rib/second thoracic ganglion), they are more exposed than any other patients to a higher risk of CH with respect to its incidence and also its severity. For this reason, these patients must be carefully selected for surgical treatment, after a complete and clear awareness of all potential risks and benefits related to the interruption of sympathetic trunk at these levels.
The target levels of the sympathetic chain that must be approached in each patient submitted to sympathectomy will be discussed in more details further ahead within this article, according to the hyperhidrosis location and considering the risk of CH, as suggested by the STS consensus (13).
The majority of patients with primary hyperhidrosis are young and healthy. This makes the preoperative preparation simpler. If the patient has no comorbidities neither relevant past medical history, complementary exams are not formally required, but we usually ask for a basic blood analysis and a simple chest X-ray.
During anamnesis, it is important to search for past lung diseases such as pneumonia, tuberculosis, pneumothorax, pleural effusion, as well as previous intrathoracic surgical interventions or any other condition that can anticipate the existence of pleuropulmonary adhesions, which could make the procedure more prone to intraoperative difficulties, although it does not constitute per se a surgical contraindication.
Actually, there are no absolute contraindications to ETS. But specific situations are closely related to worse outcomes, then their existence must be excluded before surgery in order to not compromise operative results. Obese and overweighed patients tend not to respond well after ETS and are under an increased risk of severe compensatory sweating. According to the STS consensus (13), patients should be encouraged to reach a body mass index (BMI) <28 before being considered surgical candidates. We still prefer to indicate surgery only for patients within their normal weight (BMI ≤25). Patients presenting with neurologic illnesses and/or under psychiatric treatment must be evaluated cautiously. Many medications used in these situations can lead to increased sweat production as a common side effect, and ETS shall not be indicated.
Basic surgical anatomy of the thoracic sympathetic trunk
The sympathetic chain runs beneath the parietal pleura along each side of the spine, at the level of the necks of the ribs, near the point where they articulate with the vertebrae. The chain is a whitish cord, visible and palpable in the majority of individuals. Even though excessive local fat can preclude a clear identification of the chain, the ribs are usually easy to visualize.
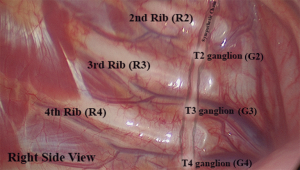
Due to its easy identification, the anatomic landmark chosen by the STS consensus (13) to guide the exact location of the interruption of the sympathetic trunk are the ribs, represented by the letter “R” (R3 = interruption at the level of the third rib).
Following the descending trajectory of the sympathetic chain on each side, the corresponding sympathetic thoracic ganglia (represented by the letter “G”) lie approximately slightly distal to each corresponding rib, at the level of the intercostal space (Figure 1). As the trunk descends, the lower ganglia tend to shift progressively downwards (13). It means that the G2 ganglion is usually located in the second intercostal space, close to the bottom of the second rib, whereas the G4 ganglion tends to lie closer to the fifth rib than to the fourth.
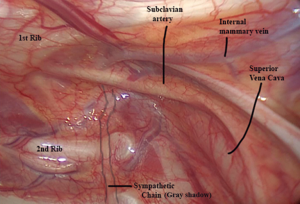
Cranially, the most relevant anatomic landmark is the first rib, which describes a “closed C-ring trajectory”. Right above its upper border, the pulsating subclavian artery can be easily found. The stellate ganglion is the most cranial part of the intrathoracic sympathetic chain and definitely must be avoided in all thoracic sympathectomies for hyperhidrosis. This important structure is not always easily seen, but lies along the axis of the trunk, immersed within the fatty tissue below the subclavian artery and the first rib. Laterally to the chain, there is only the inner face of the chest wall. Care must be taken at the lower border of the ribs, where the intercostal neurovascular bundles lie and cross the sympathetic chain underneath to it. Medial to the chain, the longus colli muscle/tendon can be identified and should not be confounded with the sympathetic chain itself. Moving towards the mediastinum even more medially, important vascular structures must be noticed (superior vena cava on the right and aorta on the left). These anatomic relations are shown in the Figure 2 (for a right sided approach) and Figure 3 (for a left sided approach).

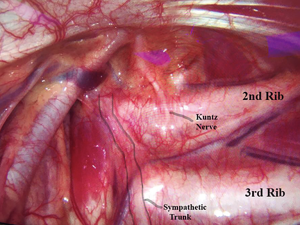
Tiny collateral fibers arising from the trunk can be present in a variable amount of patients. These are the “Kuntz nerves” (16-18), as exemplified in the Figure 4. Acting as alternative neural pathway to the brachial plexus, they can be responsible for persisting hyperhidrosis after ETS. In order to avoid that, it is advisable to extend the chain interruption in about 2 cm laterally over the inner face of the ribs until denudate the periosteum, so these small nerves can be properly transected. Some critics argue that this is not justifiable because the Kuntz nerves are not always present and this maneuver can increase postoperative pain, but we advocate extending the dissection laterally towards the ribs routinely, in order to cut the Kuntz nerves, which are not always easy to visualize, even when they are present.
Anatomical variability on the sympathetic chain anatomy is not uncommon. Aside from being observed between different individuals, differences between right and left sides can also be seen in the same patient (16). That’s why surgical inspection at the first moment of the operation must be done carefully in every patient.
Patient preparation and anesthetic considerations
Patients selected to ETS are usually operated in dorsal decubitus with abducted and fixed arms at 90 degrees. The trunk is elevated in 30–40 degrees. This elevation helps to displace the lung downwards. A gross cushion under the knees or a slight bed elevation at the knee level is useful to safely place the patients and to avoid them to slip down during the procedure. We believe this semi-fowler position offers a simple and easy way to perform the surgery bilaterally in a fast, safe and effective manner. Rotating the bed laterally one side to another is a useful maneuver to enhance surgical exposure.
Axillary hair is shaved in the most inferior portion of the axillary fossa, immediately before local antisepsis and draping.
Regarding the anesthetic management of these patients, there is no consensus and many strategies are reasonable: patients can be intubated under general anesthesia or sedated without intubation, if clinically suitable conditions are ensured (19). If the patient is intubated, normal endotracheal or double-lumen endotracheal tubes can be used (20). When using a normal endotracheal tube, it is important to have an experienced anesthesiologist who can control patient’s O2 enriched ventilation in a low-volume/high-frequency fashion, alternating brief periods of apnea when necessary. This option is absolutely safe, feasible and is, indeed, our method of choice, except in patients in whom intraoperative difficulties are preoperatively anticipated [presence of an azygos lobe (1% of population) identified in a chest X-ray, previous history of tuberculosis, pleural effusion, pneumothorax and/or reoperation]. In these situations, we prefer to use a double-lumen tracheal tube with selective lung ventilation.
Hand temperature monitoring is advisable and, in patients operated for palmar hyperhidrosis, an elevation of 1 °C in hand temperature immediately after ETS is an indicator of adequate chain interruption (21,22). An alternative to temperature monitoring, a doppler blood flow device can be used to detect a drop in the sympathetic vascular tone (22).
Antibiotics are used prophylactically, being infused as soon as the patient arrives at the operating room (first generation cephalosporin, if the patient is not allergic).
We always perform preemptive local anesthesia with long-lasting agents before initiating the procedure, at the level of the skin incisions. A complementary anesthetic intercostal block at multiple intercostal spaces is also useful, if long enough needle is available.
Postoperative analgesic regimen includes ordinary analgesics and non-steroid anti-inflammatory agents. Opioids normally are not required and no postoperative exams or X-rays are necessary, unless surgical complications are suspected. Even though some authors advocate in favor of a “fast-track” sympathectomy in an outpatient basis (23), our routine is to send patients home the following morning.
Surgical technique
If there are no anticipated intraoperative adhesions or difficulties, we advocate starting the procedure by approaching the patient’s dominant side first. So, if the patient is right-handed, we start by the right side, just because if any unexpected intraoperative complication occurs and the surgery has to be aborted, there is a chance that at least the dominant side will be treated. If there is a preoperative anticipation of adhesions or difficulties, we prefer to start the procedure by the supposedly easier side, in order to ensure that at least this side will be treated, even if problems occur when approaching the contralateral side.
The access to the pleural cavity can be obtained by several different strategies. The most common access is by two-port videothoracoscopy, which is our method of choice. We routinely use two 5 mm ports. The camera port is anterior and the incision is placed in the fourth or fifth intercostal space underneath the mammary sulcus in women (so the breast covers the scar and ensures a good postoperative cosmetic aspect) and around the areola in men, also providing excellent aesthetic results (see Figures 5-7). The second port is placed laterally at the third intercostal space, at the base of the axillary fossa, just posterior to the anterior axillary folder formed by the pectoralis major muscle.

Depending on the type and caliber of the surgical instruments available, the procedure can be carried out in a uniportal fashion (23-26). Another single-port technique recently described is the subxiphoid access (27), which offers the possibility to perform the ETS bilaterally through a unique incision in order to minimize chest wall trauma and postoperative pain. The same rationale is used to justify another possible, but unusual access which is based on embryonic natural orifice transluminal surgery (e-NOTES) technique: the transumbilical uniportal access, in which a thin and long gastroscope is introduced in the abdomen through the umbilicus and reaches both pleural cavities through two small diaphragmatic orifices—one in each side —to perform the sympathectomy (28).
CO2 insufflation can be applied (10 mmHg at maximum), but its use is mandatory only in approaches using “needlescopic” instruments (2–3 mm), when the lung collapse is harder to be obtained spontaneously.
Even though we use the term “sympathectomy” widely and almost indistinctly, there are clear differences between the possible pathways to interrupt the sympathetic chain. The real meaning of the term “sympathectomy” includes the dissection and surgical removal of a specific segment of the sympathetic trunk and ganglia. This technique was the standard of care during the era of open sympathetic surgery, which is definitely in the past. The technique is effective, but is more time-consuming, leads to higher blood loss and due to its greater magnitude, is more prone to complications. Nowadays, the real sympathectomy is practically never done, but its name has remained worldwide as a generic reference to hyperhidrosis surgical treatment, regardless the technique applied.
The most widely used technique in present days is the “sympathicotomy” or “sympathotomy”, which refers to interruption of the sympathetic trunk only by its complete transection, that can be done using electrocautery, endoscopic scissors, harmonic scalpel or other more sophisticated devices such as Vasoview© (Maquet Inc., Rastatt, Germany)—instrument originally developed for minimally invasive vessel harvesting for coronary bypass surgery (26). The harmonic scalpel is our instrument of choice, because it combines cutting and coagulating functions, allied to a significantly lower spread of local heat and less smoke production—ensuring then a lower risk of thermal lesion of surrounding structures (29). These are important features, considering the proximity of the stellate ganglion and major blood vessels to the sympathetic trunk. The sympathicotomy can be performed alone at the desired level of the trunk (see Figure 8) or in conjunction with the isolation and thermoablation of the sympathetic ganglion corresponding to the approached level, as demonstrated on the Figure 9.


Another possible thoracoscopic approach to the sympathetic chain is by the so-called endoscopic sympathetic block (ESB). This method is characterized by the interruption of the chain without transecting it, but by means of metallic clips applied across the trunk, after dissecting and isolating the sympathetic chain away from the parietal pleura and the inner thoracic wall (see Figure 10). Since the anatomical integrity of the sympathetic trunk is theoretically preserved, this technique has been introduced (33) as an alternative that could offer the theoretical advantage of being potentially reversible in cases which patients regret from surgery because of the postoperative onset of an incapacitating CH. Our practice is to propose the ESB with metallic clips only for patients with facial blushing and/or craniofacial hyperhidrosis, exploring this theoretical potential reversibility through the clips removal in a second procedure, if the patient regrets from the primary surgery. In these cases, it is crucial to emphasize and clarify to patients before the first operation that this reversibility is a hypothetical advantage, which may not become real even after the clips removal (33).

The chosen method to perform the trunk interruption will ultimately depend on the available instruments and the surgeon’s preference, since many studies (34-36) suggest that the results among all the described techniques are comparable regarding symptoms resolution, recurrence rates and incidence of CH.
After performing the sympathectomy in each side, it is important to review the local hemostasis and, if necessary, test the lungs for air leaks. Once active bleeding and air leaks are ruled out, the surgically induced pneumothorax is totally evacuated through a thin chest tube connected to a water-seal system and inserted on the camera port. When the lung is fully re-inflated, the chest tube is finally pulled out and then the wounds are closed.
How to approach the sympathetic trunk: selecting the level of interruption according to the location of hyperhidrosis
Whatever instruments and technique used, the most important aspect of the surgery is to correctly define in which level (or levels) the sympathetic trunk shall be interrupted. This decision has to be based on the patient’s symptoms localization, in balance with the risks of postoperative CH. Many publications suggest that the more cephalad the level transected (especially in G2 ganglion vicinity) and the more trunk levels are interrupted, the greater will be the risk of CH (37-41). This general rule must be taken into consideration when planning the sympathectomy and, in our opinion, patients must be fully informed about all the possibilities and should take part in the decision process, weighting all the potential benefits and risks in an individualized context.
Although there is still some controversy and conflicting opinions regarding the best level of interruption of the sympathetic trunk, the STS consensus (13) has proposed the standardized approaches as follows:
For palmar hyperhidrosis, the interruption at the R3 and R4 levels will provide the most curative scenario, marked by completely dry hands, but with a higher risk of CH. For patients who prefer a lower risk of CH at the expense of less dry hands, the interruption can be done only at R4 level. In this situation, patients must be warned that hands will get drier than before, but possibly may remain moist. Perhaps this option is suitable for patients with palmar hyperhidrosis presenting with only mild symptoms/complaints. Considering the increased risk of CH when dealing with multiple levels of the trunk, patients with important complaints of isolated palmar hyperhidrosis can also be treated by a R3 level interruption alone, with good results.
Patients with isolated plantar hyperhidrosis should not be referred to ETS. However, patients presenting with plantar complaints associated to palmar and axillar sweating are frequent in daily practice. During their first consultation, it is important to highlight that the results of ETS for the plantar sweating complaints are quite unpredictable. The response can occur or not, and if it does, it may be complete or only partial. In general, patients submitted to prior ETS do not demand for complementary lumbar sympathectomy, perhaps because, even in the absence of any substantial plantar improvement, the impact of the sweating feet is generally less significant in their QoL than the hand and axillary components. Patients with palmar and plantar hyperhidrosis can benefit from R4–R5 interruptions to ensure drier feet, but R4 level alone may offer acceptable results with possibly moister extremities, but lower CH risk.
For isolated axillary hyperhidrosis, the STS consensus recommends other treatment modalities prior to consider a sympathectomy, because this procedure is related to lower success and higher regretting rates in these patients. If less invasive therapies fail and an ETS becomes indicated, the suggested strategy is a R4–R5 interruption, which also tends to be our personal choice. The same strategy can be applied to patients with palmar-axillary and palmar-axillary-plantar hyperhidrosis.
Regardless the STS consensus, for individuals with axillar and/or plantar hyperhidrosis associated with palmar symptoms, whenever the hand sweating component presents as a major complaint and completely dry hands are considered imperative for these patients, we strongly believe that R3 interruption should be added to the originally planned approach, even though the risk of CH is higher in these cases. R3 is the level that ensures the driest hands possible. Of course, patients must agree on that, after being fully informed about the risk/benefit ratio involved in this strategy.
For facial blushing/hyperhidrosis, non-surgical therapies should be the first-line treatment. If conservative measures fail, surgical indication is preceded by an extremely judicious consultation, where the patient must be fully and clearly warned about the elevated risk of postoperative CH. This is a major concern in these cases, in which the CH is usually more severe and the regretting rates after surgery are the highest among all hyperhidrotic patients, even though the sympathectomy is indeed effective on abolishing the facial sweating/blushing symptoms. It’s important that patients ensure that the symptoms really have a deep impact on their QoL to justify the risks.
The STS consensus suggests an isolated R3 interruption or an alternative combined R2–R3 interruption. The latter is more effective, but related to a higher risk of CH. Our choice is R3 block with clips when isolated mild facial sweating is present and R2–R3 block with clips for facial blushing, if the patient is willing to accept the risk and agrees on the strategy.
Complications
ETS is a very well tolerated procedure and complications rarely occur.
The most obvious intraoperative complications possible are lung injury, pneumothorax, major bleedings, chylothorax and phrenic nerve injury. All of them are rare and can be prevented by a meticulous and careful surgical technique.
Early postoperative pneumothorax and pleural effusion requiring intervention are possible, but unlikely.
Since the use of harmonic scalpels has been introduced and the G2 ganglion approach has been practically abandoned, the incidence of Horner’s syndrome has dropped almost to zero.
Bradycardia, if occurs, is usually asymptomatic and clinically irrelevant in nearly all patients. However, some high performance professional athletes may have their physical capacity affected and must be warned about this risk before surgery.
Postoperative pain is easily controlled by regular analgesics and non-steroid anti-inflammatory drugs and is usually self-limited, disappearing within the first month after surgery. With the advent of thinner instruments and trocars, the incidence of chronic pain is extremely low.
Persistence of the symptoms or early recurrence usually indicate surgical failure, mostly related to incomplete or inadequate denervation and require reoperation in the majority of cases. Misinterpretations due to local anatomic variations, blood vessels crossing over the trunk, local pleural thickening or adhesions are among the possible causes, in addition to technical faults. Approaching the sympathetic trunk at wrong levels can also lead to unsatisfactory early postoperative results.
Late recurrence is not common but can occur, probably by nerve regeneration, prone to happen when the transected trunk ends remain close to each other or in children who still might grow after surgery. Both situations also may require reoperation.
Re-sympathectomy is indicated using double-lumen endotracheal tubes, generally providing successful resolution of persistent/recurrent symptoms, but the risk of worsening a possible CH that has emerged after the first surgery shall not be neglected.
CH
CH is being addressed separately from the complications due to its importance and also because, in our opinion, it is more a possible undesirable effect of the sympathectomy than a surgical complication itself.
CH can be defined as an onset of a new pattern of excessive sweating after surgery, to the point of being noticed and become a problem to the patient.
Its real incidence is extremely difficult to precise and varies in the literature from 3% to 98% (13). This wide variation is explained by several factors related to individual and geographic aspects, the surgical technique and the lack of a unified nomenclature about the definition of CH and its severity grades. In the end, the patient’s perception is what counts the most to establish the diagnosis.
It can occur anywhere on the body, but abdomen, thorax, back, thighs and/or groin regions are more likely to be affected.
As mentioned earlier, there is no consensus regarding CH grades, but a simple classification is to CH categorize as mild, moderate and severe (42). Mild CH is characterized by small amount of sweat, which doesn’t flow and is tolerable, being initiated by usual triggers—ambient heat, stress, physical activity—without embarrassing the patient, nor requiring to change of clothes. In moderate CH, the sweat amount is higher and coalesces into droplets that flow. The triggers are the same and even though it’s uncomfortable, it does not embarrass the patient nor requires changing clothes. Severe CH occurs when the sweat amount is large, flowing profusely and requiring one or more change of clothes. The above-mentioned triggers can be minimal or absent and the situation is definitely embarrassing, determining a near miserable QoL, compromising social and professional activities.
Gustatory sweating is an uncommon but curious situation that can occurs in up to 1/3 of patients. It is characterized by increased facial sweating under certain stimulus such as ingestion of sour/spicy foods and drinks.
It is impossible to predict who will develop CH and in which grade, but as mentioned in the text, the risk is increased in some situations. So, avoiding the trunk interruption at levels around G2 ganglion and limiting the number of interruption levels are the best “prevention” methods. Contraindicating surgery in overweight/obese patients is also helps to reduce the risk of CH occurrence and severity.
If CH occurs after a sympathetic block, clips removal can be attempted to reverse the symptoms, but literature is not consistent about this potential reversibility (33) and patients must be warned that this is only a theoretical possibility. There are evidences in the literature (43) suggesting that the best chances for a successful reversal are dependent on a clip removal early after the endoscopic blocking (ideally within two weeks). This might be explained by the crescent fibrosis/necrosis that the clips promote in the sympathetic trunk over the time.
Clinical experience with sympathetic chain reconstruction using nerve interposition/grafts is still very limited to a few centers and the results are too fragile and inconsistent to support its indication in daily clinical practice (44-46). Further investigation is still needed, but reconstruction strategies probably will gain more value in the future.
Medical management of CH is not curative, but it can contribute significantly to reduce patient’s discomfort (42). Multidisciplinary measures such as keeping strict weight control and following a non-thermogenic diet are strongly advisable; among other general recommendations on avoiding excessively hot environments, maintaining appropriate physical activities and selecting/using adequate “breathable” fabrics/clothes. The combined use of substances originally indicated for medical treatment of primary hyperhidrosis also may play an important role and drugs such as anticholinergic agents (oxybutynin), benzodiazepines and beta-blocker agents can be useful, whereas other sweat-stimulating drugs should be avoided (alcohol and antidepressants, among many others). Depending on the extent/location of the sweating complaints, topical preparations and antiperspirants can also be utile, as well as botulinum toxin injections.
Long-term assessments: follow-up, QoL and results
Follow-up
Late recurrences are more frequent in the first five years after surgery and the postoperative sweating patterns—particularly CH complaints—can suffer seasonal influences over the time. For these reasons, patient’s follow-up should be done ideally in the long-term. We recommend postoperative revisions starting at 15 days after surgery, followed by 1 month, 3, 6, 12 months, and then yearly for at least five years.
QoL after sympathectomy
QoL measurements are crucially important in hyperhidrosis patients, since their pathology and treatment deeply affect all its components, including physical, functional, psychological, emotional, social and professional aspects (47). Generic QoL questionnaires exist and some have been adjusted to hyperhidrosis (48,49), but QoL questionnaires truly dedicated/designed for hyperhidrosis patients (50,51), such as the proposed by de Campos and coworkers (51) are much better tools because they evaluate more precisely all above mentioned aspects (and many others) with specific focus on hyperhidrosis and sympathectomy, in addition to possibly standardize this assessment method internationally. When applied at regular intervals, starting before the first preoperative consultation, they enable an evolutional analysis of QoL data after treatment and along the postoperative follow-up time. In his series (51), de Campos has demonstrated that, although small differences exist between QoL measured at 30 days and at 5 years after surgery (92% and 90.6% of patients reporting better QoL if compared to before surgery, respectively), the long-term preservation of the substantial improvement in QoL observed early after the surgery is evident. As the follow-up continues, further analysis indicate that at 10 years after surgery, the QoL improvement still remains, with 90.7% of patients reporting better QoL, if compared to the pre-treatment period.
Postoperative results
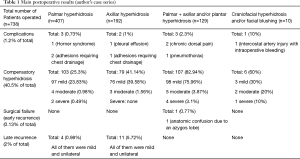
From June 1998 to December 2016, our group has performed 739 sympathectomies for primary hyperhidrosis in 738 patients. From these patients, 427 were female (57.9%) and 311 were men (42.1%), with a mean age of 24.4 years (ranging from 12 to 66 years).
In this series, 407 patients had palmar hyperhidrosis (55.1%), while 192 had isolated axillary symptoms (26%) and 129 (17.5%) had mixed complaints (palmar + axillary and or plantar hyperhidrosis). Only ten patients presented with craniofacial complaints (1.4%): seven reporting isolated sweating and three with isolated facial blushing. In these patients with craniofacial symptoms, the technique used was sympathetic block using metallic clips.
All patients with axillary isolated hyperhidrosis submitted to surgery have tried—and failed—at least two forms of non-surgical treatment before surgical consultation.
Operative mortality was zero. The global incidence of surgical complications was 1.2%. CH cases were excluded from complications list and have been addressed separately (see next paragraph). Among all patients, three of them had unsuspected pleural adhesions (0.4%) and required chest tube drainage at the end of the procedure, with tubes being removed in the second postoperative day. In a patient with facial blushing, one intraoperative bleeding (0.13%) occurred (injury of the third intercostal artery by the metallic clip) and it was easily controlled thoracoscopically also with metallic clips. One pneumothorax was diagnosed (0.13%), requiring chest tube insertion. This one was detected by persistent air leak at the end of the procedure, although no evident lung injury was seen. Tube was pulled out at the first postoperative day, 12 hours after air leak cessation. One case of postoperative pleural effusion was diagnosed (0.13%) by chest X-ray fifteen days after surgery and was treated by simple thoracentesis. One case of transient left Horner’s syndrome was reported (0.13%) in a patient with palmar hyperhidrosis. Palpebral ptosis has resolved spontaneously after three weeks. Chronic dorsal pain was reported by two patients with palmar-axillar-plantar hyperhidrosis (0.27%).
The global incidence of CH was 40.5%, but only one patient regretted from surgery because a severe CH developed early after the ESB. He had the clips removed 35 days after the ESB, with only partial resolution of the CH, which moved from severe to moderate. It is interesting to mention that the clips removal did not change the resolution of the facial hyperhidrosis, which was totally cured after the ESB.
One surgical failure (0.13%) was reported on the right, due to an azygos lobe that was not properly identified in a palmar-axillar-plantar hyperhidrosis patient. This procedure was carried out under general anesthesia and with a normal endotracheal tube. Once the failure has been detected, reoperation with double-lumen tracheal tube was scheduled and performed unilaterally after 14 days, under total lung collapse, and the failure was corrected.
We had fifteen late recurrences (2%). Four were observed in palmar hyperhidrosis patients and eleven in axillar patients. All were mild and unilateral in the non-dominant side of the body; so all patients have decided not to be operated again.
The mean follow-up time was 32 months (ranging from 9 to 63 months) and our main results are summarized on the Table 1.
Full table
Regarding satisfaction after surgery, the patients were divides into three groups: “Very satisfied” (group A—absolutely satisfied, with significant improvement in QoL after surgery and with no complaints), “Satisfied” (group B—satisfied and with improvement in QoL after surgery, but with minor complaints) and “Unsatisfied” (group C—major complains with same or worse QoL after surgery). All patients were asked to put themselves in one of these groups in each follow-up consultation. Comparing the three groups at 30 days, 1 year and 2 years after surgery, we have observed a slight but progressive drop in the “very satisfied patients” group. Parallel to this, the ratio of patients reporting to be in the “Unsatisfied” group has increased in the same period. However, during these first two years after surgery, the percentage of patients reporting to be “non-unsatisfied”—those who reported to satisfied or very satisfied (groups A + B)—remained meaningfully high, as shown in Table 2.

Full table
Summary and conclusions
Patients who suffer from primary hyperhidrosis frequently experiment a miserable QoL.
Thoracic sympathectomy and its variations are the most valuable tools in the curative treatment these patients.
Those presenting with palmar hyperhidrosis are the most suitable to surgery as first-line treatment, in which the best results and grades of satisfaction are observed, with a significant upgrade in their QoL.
For patients with axillar, plantar and/or facial complaints, sympathectomy can also play an important role and obtain good results in symptoms relief and in QoL improvement. However, the selection of patients for surgery must be even more judicious in these cases, and the surgical treatment should be indicated only after other less invasive modalities of treatment fail.
When planning the surgical approach to the sympathetic trunk, two main aspects have to be considered: the location of the symptoms and the risk of postoperative CH. Patients should be fully warned about all benefits and risks, being encouraged to take part in the decision process.
The surgery is performed by means of minimally invasive approaches and the complication’s rates are very low.
Nevertheless, the occurrence of CH should never be ignored and all maneuvers to prevent its occurrence must be pursued as much as possible during the procedure: lowering the level of interruption and limiting the extent of manipulation on the sympathetic trunk seems to lower the incidence and severity of compensatory sweating.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1. Alexander W. The treatment of epilepsy. Edinburgh: Young J Pentland, 1889: 27-106.
- Kotzareff A. Resection partielle du tronc sympathique cervical droit pour hyperhidrose unilaterale. Rev Med Suisse Rom 1920;40:111-3.
- Hughes J. Endothoracic sympathectomy. Proc R Soc Med 1942;35:585-6. [PubMed]
- Ro KM, Cantor RM, Lange KL, et al. Palmar hyperhidrosis: evidence of genetic transmission. J Vasc Surg 2002;35:382-6. [Crossref] [PubMed]
- Bovell DL, Clunes MT, Elder HY, et al. Ultrastructure of the hyperhidrotic eccrine sweat gland. Br J Dermatol 2001;145:298-301. [Crossref] [PubMed]
- Minor V. Ein neues Varfahren zu der klinischen Untersuchung der Schwei absonderung. Z Neurol 1928;101:302-8.
- Low PA, Caskey PE, Tuck RR, et al. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol 1983;14:573-80. [Crossref] [PubMed]
- Heckmann M, Plewig G. Low-dose efficacy of botulinum toxin A for axillary hyperhidrosis: a randomized, side-by-side, open label study. Arch Dermatol 2005;141:1255-9. [Crossref] [PubMed]
- Lang E., Foerster A, Pfannmuller D, et al. Quantitative assessment of sudomotor activity by capacitance hygrometry. Clin Auton Res 1993;3:107-15. [Crossref] [PubMed]
- Kurta AO, Glaser DA. Emerging Nonsusgical treatments for Hyperhidrosis. Thorac Surg Clin 2016;26:395-402. [Crossref] [PubMed]
- Lillis PJ, Coleman WP. Liposuction for treatment of axillary hyperhidrosis. Dermatol Clin 1990;8:479-82. [PubMed]
- Wollina U, Kostler E, Schonlebe J, et al. Tumescent suction curettage of sweat glands versus minimal skin resection with subcutaneous curettage of sweat glands in axillary hyperhidrosis. Dermatol Surg 2008;34:709-16. [PubMed]
- Cerfolio RJ, De Campos JR, Bryant AS, et al. The Society of Thoracic Surgeons Expert Consensus for the Surgical Treatment of Hyperhidrosis. Ann Thorac Surg 2011;91:1642-8. [Crossref] [PubMed]
- Kao MC, Lee WY, Yip KM, et al. Palmar hyperhidrosis in children: treatment with video endoscopic laser sympathectomy. J Pediatr Surg 1994;29:387-91. [Crossref] [PubMed]
- Bell D, Jedynak J, Bell R. Predictors of outcome following endoscopic thoracic sympathectomy. ANZ J Surg 2014;84:68-72. [Crossref] [PubMed]
- Zhang B, Li Z, Yang X, et al. Anatomical variations of the upper thoracic sympathetic chain. Clin Anat 2009;22:595-600. [Crossref] [PubMed]
- Ramsaroop L, Singh B, Moodley J, et al. Anatomical basis for a successful upper limb sympathectomy in the thoracoscopic era. Clin Anat 2004;17:294-9. [Crossref] [PubMed]
- Ramsaroop L, Partab P, Singh B, et al. Thoracic origin of a sympathetic supply to the upper limb: the 'nerve of Kuntz' revisited. J Anat 2001;199:675-82. [Crossref] [PubMed]
- Mier-Odriozola JM. Simpatectomía torácica bilateral R3-R4 en un paciente sedado. Gac Med Mex 2016;152:228-30. [PubMed]
- Toolabi K, Aminian A, Javid MJ, et al. Minimal access mediastinal surgery: One or two lung ventilation? J Minim Access Surg 2009;5:103-7. [Crossref] [PubMed]
- Liu Y, Li H, Zheng X, et al. Sympathicotomy for palmar hyperhidrosis: the association between intraoperative palm temperature change and the curative effect. Ann Thorac Cardiovasc Surg 2015;21:359-63. [Crossref] [PubMed]
- Li X, Tu YR, Lin M, et al. Minimizing endoscopic thoracic sympathectomy for primary palmar hyperhidrosis: guided by palmar skin temperature and laser doppler blood flow. Ann Thorac Surg 2009;87:427-31. [Crossref] [PubMed]
- Obeso Carillo GA, Cañizares Carretero MÁ, Padin Barreiro L, et al. Nonintubated bilateral single port thoracoscopic sympathectomy in the context of an outpatient program, the least invasive management for hyperhidrosis surgery. Ann Transl Med 2015;3:357. [PubMed]
- Karamustafaoglu YA, Kuzucuoglu M, Yanik F, et al. 3-year follow-up after uniportal thoracoscopic sympathicotomy for hyperhidrosis: undesirable side effects. J Laparoendosc Adv Surg Tech A 2014;24:782-5. [Crossref] [PubMed]
- Yang Y, Zeng L, An Z, et al. Minimally invasive thoracic sympathectomy for palmar hyperhidrosis via a single unilateral incision approach by the pleura videoscope. J Laparoendosc Adv Surg Tech A 2014;24:328-32. [Crossref] [PubMed]
- Ng CS, Lau RW, Wong RH, et al. Single-port vasoview sympathectomy for palmar hyperhidrosis: a clinical update. J Laparoendosc Adv Surg Tech A 2014;24:32-4. [Crossref] [PubMed]
- Chen JT, Liao CP, Chiang HC, et al. Subxyphoid single-incision thoracoscopic bilateral ablative sympathectomy for hyperhidrosis. Interact Cardiovasc Thorac Surg 2015;21:119-20. [Crossref] [PubMed]
- Zhu LH, Du Q, Chen L, et al. One-year follow-up period after transumbilical thoracic sympathectomy for hyperhidrosis: outcomes and consequences. J Thorac Cardiovasc Surg 2014;147:25-8. [Crossref] [PubMed]
- de Campos JRM, Wolosker N, Yasbek G, et al. Comparison of pain severity following video-assisted thoracoscopic sympathectomy: electric versus harmonic scalpels. Interact Cardiovasc Thorac Surg 2010;10:919-22. [Crossref] [PubMed]
- Vannucci F, Araújo JA. Right sided endoscopic thoracic sympathectomy (ETS) at R3 level for palmar hyperhidrosis using harmonic scalpel (author’s personal archive). Asvide 2017;4:152. Available online: http://www.asvide.com/articles/1457
- Vannucci F, Araújo JA. Left sided endoscopic thoracic sympathectomy (ETS) at R3–R4 level with T3 ganglion thermoablation for palmar hyperhidrosis using harmonic scalpel (author’s personal archive). Asvide 2017;4:153. Available online: http://www.asvide.com/articles/1458
- Vannucci F, Araújo JA. Left sided endoscopic sympathetic block (ESB) using metallic clips at R3 level for palmar hyperhidrosis (author’s personal archive). Asvide 2017;4:154. Available online: http://www.asvide.com/articles/1459
- Lin CC, Mo LR, Lee LS, et al. Thoracoscopic T2-sympathectomy block by clipping – a better and reversible operation for treatment of hyperhidrosis palmaris: experience with 326 cases. Eur J Surg Suppl 1998;580:13-6. [PubMed]
- Coelho MS, Silva RF, Mezzalira G, et al. T3T4 endoscopic sympathetic blockade versus T3T4 video thoracoscopic sympathectomy in the treatment of axillary hyperhidrosis. Ann Thorac Surg 2009;88:1780-5. [Crossref] [PubMed]
- Hwang JJ, Kim DH, Hong YJ, et al. A comparison between two types of limited sympathetic surgery for palmar hyperhidrosis. Surg Today 2013;43:397-402. [Crossref] [PubMed]
- Inan K, Goksel OS, Uçak A. Thoracic endoscopic surgery for hyperhidrosis: comparison of different techniques. Thorac Cardiovasc Surg 2008;56:210-3. [Crossref] [PubMed]
- Ishy A, de Campos JR, Wolosker N, et al. Objective evaluation of patients with palmar hyperhidrosis submitted to two levels of sympathectomy: T3 and T4. Interact Cardiovasc Thorac Surg 2011;12:545-8. [Crossref] [PubMed]
- Yang J, Tan JJ, Ye GL, et al. T3/T4 thoracic sympathictomy and compensatory sweating in treatment of palmar hyperhidrosis. Chin Med J (Engl) 2007;120:1574-7. [PubMed]
- Weksler B, Blaine G, Souza ZB, et al. Transection of more than one sympathetic chain ganglion for hyperhidrosis increases the severity of compensatory hyperhidrosis and decreases patient satisfaction. J Surg Res 2009;156:110-5. [Crossref] [PubMed]
- Araújo CA, Azevedo IM, Ferreira MA, et al. Compensatory sweating after thoracoscopic sympathectomy: characteristics, prevalence and influence on patient satisfaction. J Bras Pneumol 2009;35:213-20. [Crossref] [PubMed]
- Bryant AS, Cerfolio RJ. Satisfaction and compensatory hyperhidrosis rates 5 years and longer after video-assisted thoracoscopic sympathotomy for hyperhidrosis. J Thorac Cardiovasc Surg 2014;147:1160-1163.e1. [Crossref] [PubMed]
- Lyra Rde M, Campos JR, Kang DW, et al. Guidelines for the prevention, diagnosis and treatment of compensatory hyperhidrosis. J Bras Pneumol 2008;34:967-77. [PubMed]
- Hynes CF, Yamaguchi S, Bond CD, et al. Reversal of sympathetic interruption by removal of clips. Ann Thorac Surg 2015;99:1020-3. [Crossref] [PubMed]
- Telaranta T. Secondary sympathetic chain reconstruction after endoscopic thoracic sympathicotomy. Eur J Surg Suppl 1998;580:17-8. [PubMed]
- Haam SJ, Seung YP, Paik HC, et al. Sympathetic nerve reconstruction for compensatory hyperhidrosis after sympathetic surgery for primary hyperhidrosis. J Korean Med Sci 2010;25:597-601. [Crossref] [PubMed]
- Rantanen T, Telaranta T. Long-Term effect of endoscopic sympathetic nerve reconstruction for side effects after endoscopic sympathectomy. Thorac Cardiovasc Surg 2016. [Epub ahead of print]. [PubMed]
- de Campos JR, da Fonseca HV, Wolosker N. Quality of Life Changes Following Surgery for hyperhidrosis. Thorac Surg Clin 2016;26:435-43. [Crossref] [PubMed]
- Kumagai K, Kawase H, Kawanishi M. Health-related quality of life after thoracoscopic sympathectomy for palmar hyperhidrosis. Ann Thorac Surg 2005;80:461-6. [Crossref] [PubMed]
- Fredman B, Zohar E, Shachor D, et al. Video-assisted transthoracic sympathectomy in the treatment of primary hyperhidrosis: friend or foe? Surg Laparosc Endosc Percutan Tech 2000;10:226-9. [Crossref] [PubMed]
- Amir M, Arish A, Weinstein Y, et al. Impairment in quality of life among patients seeking surgery for hyperhidrosis (excessive sweating): preliminary results. Isr J Psychiatry Relat Sci 2000;37:25-31. [PubMed]
- de Campos JR, Kauffman P, Werebe Ede C, et al. Quality of life, before and after thoracic sympathectomy: report on 378 operated patients. Ann Thorac Surg 2003;76:886-91. [Crossref] [PubMed]