Thoracoscopic thymectomy: technical pearls to a 21st century approach
Introduction
Traditionally, thymectomies have been performed from either a median sternotomy (1,2) or via a transcervical approach (3). The median sternotomy and variants (e.g., inverted T, mini-thoracoscopic) have been employed for both myasthenia gravis patients and for those with thymoma. The transcervical approach has been mainly reserved for those with myasthenia gravis. While the transcervical approach is less invasive than a sternotomy, it leaves a rather large incision and the visualization can be less than that achieved with a sternotomy. Over the past decade, as both instrumentation and our technical proficiency have advanced, we have transitioned from a median sternotomy approach to a video-assisted thoracoscopic surgery (VATS) approach to thymectomy for small thymoma and myasthenia gravis patients. The aim of this study was to reflect on our evolution in order to identify specific technical points that enable safe, efficacious VATS thymectomy.
Methods
We performed a retrospective review of our prospectively maintained Institutional Review Board approved Outcomes database. We identified all patients who underwent thymectomy, via either sternotomy or VATS approach who did not require major vascular reconstruction. We measured length of stay, chest tube duration, and major complications. Results are reported as percent or mean ± standard deviation when appropriate. Comparative analyses were performed with the chi-square test for categorical variables and the t-test for continuous variables. A two-sided level of significance was used with alpha =0.05. Statistical analyses were performed with JMP for Windows version 8 (SAS Institute, Inc., Cary, NC USA).
Results
Patients’ records that underwent thymectomy from January 1, 2001 through April 1, 2010 were reviewed. We identified 38 patients. Twenty-four underwent sternotomy and 14 VATS. There were 29 women and 9 men. The mean length of stay was significantly shorter in the VATS cohort at 2.3±1.2 days as compared with 4.3±2.9 days with the sternotomy approach (Table 1). There was no difference between approaches in the duration of chest tube drainage postoperatively. There was a trend toward less frequent complications in the VATS approach as compared with the sternotomy approach (Tables 1,2). However, given our small sample size this did not reach significance.
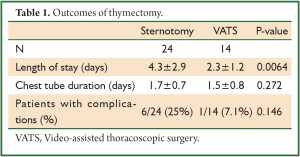
Full table
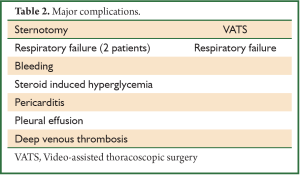
Full table
Operative approach
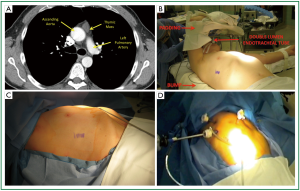
A representative chest CT cut of an ideal patient is provided (Figure 1). The lesion is less than 3 cm, does not demonstrate any pericardial or great vessel involvement. The patient is intubated with a double lumen endotracheal tube. The lung isolation provides excellent much needed visualization. We position the patient in a partial left lateral decubitus position with the right arm in a swimmers position (Figure 1B). A bump is placed under the right chest to provide access to the axilla. It is crucial to pad the right arm well to avoid ulnar nerve palsy. We scrub, prep, and drape both hemithoraces widely (Figure 1C). This draping affords us the opportunity to access the left chest if needed. We have a separate video camera and scope setup available in case there is need for added visualization of the left pleura and dissection near the left phrenic nerve. On the right hemithorax we typically place one 12-mm and two 5-mm trocars. The location of these trocars is seen in Figure 1D. We have 5-mm scopes available. The first trocar is placed under direct cut down and the subsequent ones under direct visualization.
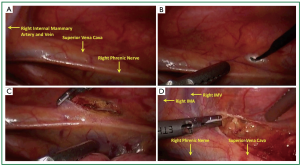
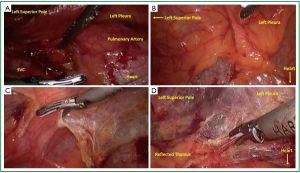
Carbon dioxide insufflation is used to help collapse the lung and to aid in dissection of areolar tissues. The contribution of the carbon dioxide cannot be understated. The right lung is retracted posteriorly and the right most extent of dissection is identified. The right phrenic nerve is seen coursing superior and lateral on the superior vena cava (Figure 2A). The internal mammary vein and artery are the superior extent of dissection. The mediastinal pleura is entered with electrocautery (Figure 2B). The pleura is incised superiorly parallel to the phrenic nerve. Electrocautery and harmonic scalpel are used as they both have advantages in dissection. The harmonic scalpel can seal and transect larger vessels. The blade of the harmonic scalpel and the hook electrocautery are complementary for dissecting various tissue planes. When the internal mammary vessels are reached, the harmonic scalpel is changed from the right to the left hand to aid in perpendicular dissection transversely (Figure 2D) across the superior mediastinum anterior to the innominate vein. The lateral to medial dissection is continued as far as visualization permits.
Attention is then turned to the inferior border at the pericardial reflection (Figure 3A). The dissection again proceeds lateral to medial across the midline. When the left pleura and the left phrenic nerve are reached the dissection is directed cephalad parallel to the nerve (Figure 3B). As one reaches the left pleural reflection, the addition of separate thoracoscope through a 5-mm trocar in the left chest (e.g., 4th or 5th intercostal space) can aid in visualization to prevent inadvertent injury to the phrenic nerve. Low level CO2 insufflation aids in compression of the left lung. CO2 on the right or ventilation should be adjusted to maintain oxygenation and adequate hemodynamics. Once the pleural dissection has been completed in circumferential nature (Figure 3C), the thymus is mobilized and retracted laterally and dissected off of the underlying pericardium (Figure 3D).
The thymus is then retracted anteriorly toward the sternum and to left lateral position as dissection proceeds to identify and free the thymus from the innominate vein (Figure 4A). Small vascular structures can be taken with electrocautery or harmonic scalpel. Dissection is performed in a circumferential fashion as allowed with gentle rolling reflection of the thymus providing adequate counter traction for dissection. Larger perforating branches from the innominate (Figure 4B) or the internal mammary vessels should be circumferentially dissected. Endoscopic ligature has been adequate to control these vessels. When space allows, we routinely clip 3 times proximally and once distal and sharply transect (Figure 4C). When the left superior thymic pole is reached, gentle caudad traction helps bring tissue into view (Figure 4D).
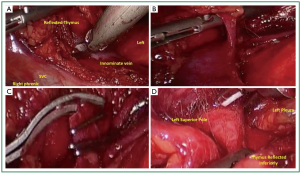
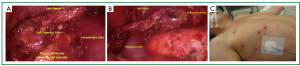
The left superior pole is often the last structure to be freed (Figure 5A). The 10-mm scope is then exchanged for a 5-mm scope. The thymus and surrounding fatty tissue that has been dissected free is placed into an endocatch bag which is brought through the 10-mm trocar. The specimen is removed and examined on the back table. A final check for hemostasis is performed and a clean dissection field is visualized (Figure 5B). A small chest tube (often a 19 French fluted drain) is placed in the anterior mediastinum and secured. The carbon dioxide insufflation is ceased and the right lung ventilated under direct visualization ensuring that all lobes are adequately expanded. The trocar sites are closed and sealed with dermal sealant (Figure 5C). The patient is recovered and extubated on the operating theater table. A postoperative chest X-ray is standard.
Discussion
Our increased ability to perform minimally invasive surgery spurned our interest in broadening our approach to thymectomy. We feel that a VATS approach to thymectomy for small (<3 cm) masses and for the myastenic patients enhances the patient experience. The convalescence is shorter and undoubtedly less uncomfortable.
In 2010 Youssef and colleagues from Seattle described their initial experience of 8 minimally invasive thymectomies and 8 traditional sternotomy thymectomies (4). They demonstrated similar results. The length of stay for the minimally invasive thymectomies was significantly shorter at 2.4 days as compared with 4.3 days for the sternotomies (P=0.001). Our results are almost identical. The approaches for the minimally invasive thymectomies that Youssef et al. employed are slightly different that ours, but the end the same.
In our VATS thymectomy approach we have found several points that we feel enhanced the conduct of the operation and allow for safe operation. The use of the harmonic scapel has been beneficial. This was described by Soon et al. (5) and we feel that it is an excellent adjunct to the electrocautery. We expand on the operative details and the technical aspects identified below.
Visual inspection of the anterior mediastinum with the video thoracoscope as well as careful comparison of the intraoperative anatomy and tissue identified to preoperative imaging are necessary to discern completeness of resection. The entire anterior mediastinum should be visualized for ectopic thymic tissue. Careful intraoperative manipulation and universal use of endoscopic bag for retrieval help to minimize breach of capsule.
Technical aspects
Patient positioning
The initial patient positioning is crucial. The double lumen endotracheal tube and partial lateral positioning facilitate excellent visualization.
Thoracoscopy
A separate video camera and thoracoscope setup are critical in the event that extra visualization of the left phrenic nerve is needed. Both 5- and 10-mm thoracoscopes with angled bevels are routinely used and necessary.
Carbon dioxide insufflation
Carbon dioxide insufflation has several benefits. The positive pressure (10 or 12 mmHg) aids in lung collapse, even in the setting of a dual lumen tube. The gas aids in dissection of areolar tissue. This passive gas dissection improves visualization of tissue plains and separation of tissues. The carbon dioxide is inert and readily absorbed.
Energy source dissection
The use of the electrocautery and harmonic scalpel are complementary. The hook cautery allows for surface pencil-type cautery. In addition, the angled nature aids in dissection around vessels. The blade dissector quality of the harmonic scalpel allows improved linear dissection. The ability to coagulate larger structures is an advantage as well. One must be mindful of the temperature of the tip, however.
Anatomic boundary delineation
An initial delineation of anatomic boundaries and consistent reassessment of the anatomy keeps the dissection moving forward and keeps from errant entry into adjacent structures or from iatrogenic injury to the left phrenic nerve.
Endoscopic ligatures
Vascular branches from the innominate vein and from the internal mammary vessels are adequately controlled without concern for hemostasis with endoscopic clip ligature. If there is question, intracorporeal suture ligature is readily performed, even with small (5-0) monofilament.
Conclusions
With proper patient selection, preoperative planning, and a standardized approach to operative conduct, we are routinely able to employ this thoracoscopic approach for complete removal of thymic tissue. This approach is ideally suited for patients with myasthenia gravis and those with small (<3 cm) thymic masses. A standard approach to dissection in thoracoscopic thymectomy streamlines the procedure and enables safe resection.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Detterbeck FC, Scott WW, Howard JF Jr, et al. One hundred consecutive thymectomies for myasthenia gravis. Ann Thorac Surg 1996;62:242-5.
- Miller JI, Mansour KA, Hatcher CR Jr. Median sternotomy T incision for thymectomy in myasthenia gravis. Ann Thorac Surg 1982;34:473-4.
- Shrager JB, Deeb ME, Mick R, et al. Transcervical thymectomy for myasthenia gravis achieves results comparable to thymectomy by sternotomy. Ann Thorac Surg 2002;74:320-6; discussion 326-7.
- Youssef SJ, Louie BE, Farivar AS, et al. Comparison of open and minimally invasive thymectomies at a single institution. Am J Surg 2010;199:589-93.
- Soon JL, Agasthian T. Harmonic scalpel in video-assisted thoracoscopic thymic resections. Asian Cardiovasc Thorac Ann 2008;16:366-9.


