Results of management of esophageal and GE junction malignancies in Nepalese context
Introduction
Esophageal cancer is the seventh largest source of cancer-related deaths across the world, with 482,300 new cases annually and 406,800 deaths (1). Smoking and excessive alcohol consumption account for about 90% of the total cases of squamous cell carcinoma of the esophagus (2). By contrast, smoking, obesity, and gastroesophageal reflux disease are thought to be the major risk factors for adenocarcinoma. Over the past few decades, the incidence of adenocarcinoma of the gastroesophageal junction (GEJ) has risen dramatically in western countries (3,4).
Despite endoscopic screening and advances in multimodality therapy, the prognosis of these tumors remains poor, with an overall 5-year survival rate of approximately 20% (5).
Not much has been explored about its management in Nepal. The aim of this study was to conduct a retrospective review of patients with esophageal and GEJ cancer undergoing surgery or combined modality treatment at BP Koirala Memorial Cancer Hospital (BPKMCH), Nepal.
Methods
A retrospective review of patients of esophageal and GE Junction (GEJ) cancer treated at our hospital was done. Dysphagia was considered as the main presenting symptom and it was graded from 0 to IV (Gr 0 - normal swallowing, Gr I - dysphagia to solid, Gr II - dysphagia to semisolid, Gr III - partial dysphagia to liquids and Gr IV - complete dysphagia). Diagnostic work-up included routine blood tests, upper GI endoscopy, Bronchoscopy (for upper esophageal and some middle esophageal lesions), CT of chest and abdomen, spirometry, ECG and echocardiography.
Inclusion criteria
Clinico-radiologically resectable cases at the time of surgery (<cT4b at the time of primary surgery or after neoadjuvant treatment), ECOG: 0-2 and M0 patients were included.
Treatment protocol
On the basis of CT findings, the tumors were divided into localized (<cT4) or locally advanced (cT4). The later was decided on the basis of CT findings of invasion into the adjacent organs. Patients with localized tumors were directly subjected to the surgery. In case of squamous cell carcinoma (SCC) with negative margins, the patients were simply followed-up every 4 months for first 2 years and then every 6 months for next 3 years. Patients with advanced tumors were subjected to preoperative concurrent chemoradiation or preoperative chemotherapy. After 4-6 weeks, patients were reassessed and if there was a response (<cT4b), they were subjected to surgery and only these patients were included in the present study.
For adenocarcinoma of GEJ or in the case of R1/R2 resection, patients were considered for adjuvant chemoradiation/ chemotherapy.
Surgical technique
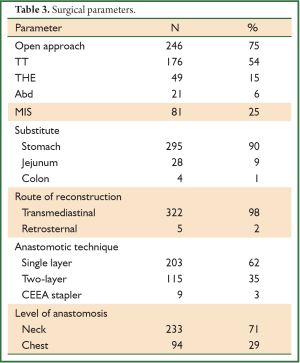
Various surgical approaches were used (Figure 1). Since year 2010, minimally invasive approach is being used more often.
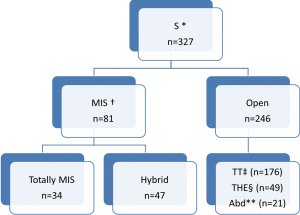
In TT technique, Mckeown’s three-incision esophagectomy for upper and middle third tumors was preferred whereas Ivor-Lewis and thoracoabdominal approaches were preferred for distal third tumors. THE was preferred for GEJ Siewert’s type II tumors. Abdominal only (laparotomy) was used for some GEJ - II and GEJ - III tumors.
In totally MIS technique, esophagus was mobilized by VATS and stomach by laparoscopy (for tumors of esophagus and GEJ-I). But GEJ-II tumors were approached by laparoscopic transhiatal route. During a hybrid MIS, one of the components of the procedure was open conventional incision (VATS-laparotomy or Thoracotomy- laparoscopy).
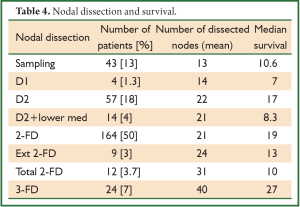
Similarly nodal dissection varied from sampling, D1, D2, two-field lymphadenectomy (2-FD), extended 2-FD, total 2-FD and three-field lymphadenectomy (3-FD). In a conventional 2-FD, infra carinal (levels 107, 108, 110, 111, 112, 109 L and 109 R) and upper abdominal nodes (levels 1, 2, 7, 8, 9, 10, 11 and 12) were dissected. In extended 2-FD, nodes along left recurrent laryngeal nerve (106 rec left) were dissected as well. In total 2-FD, nodes along both recurrent laryngeal nerves were dissected as well. During 3-FD, nodes of both sides of neck were dissected along with total 2-FD. Final pathological staging was done using 7th edition UICC system (6).
Statistical analysis
Statistical analysis was done using SPSS 16.0. Categorical variables were analyzed using Chi2 test and numerical variables analyzed using t-test. Kaplan-Meier curve was used for survival analysis. Survival curves were compared with log-rank test. P value less than 0.05 was considered to be statistically significant.
Results
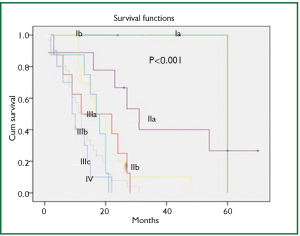
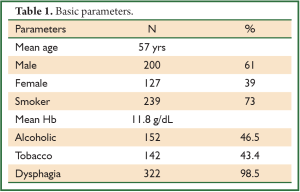
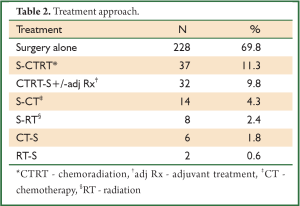
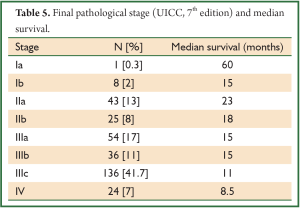
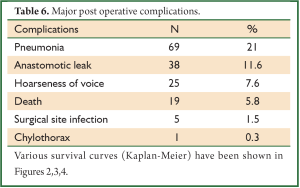
In a period of last 11 years, about 1,350 patients with the diagnosis of esophageal and GEJ cancer were treated in our hospital. 327 patients received surgical treatment. Patients had upper, middle esophageal, GEJ-I, GEJ-II and GEJ-III tumors in 24 (7%), 99 (31%), 75 (23%), 121 (37%), and 8 (2%) cases, respectively. Grade 0, I, II, III and IV dysphagia was present in 1.5%, 1.8%, 23.9%, 64.2% and 8.6%, respectively. Weight loss varied from 0-25 kg with mean weight loss of 9.3 kg in last three months of presentation. Basic parameters, Treatment approaches, surgical parameters, nodal dissection, final stages and major post - operative complications have been shown in Tables 1-6, respectively.
Full table
Full table
Full table
Full table
Full table
Full table
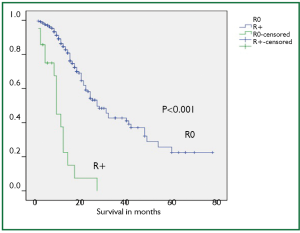
Mean post operative stay, operative time, tumor length and intra operative blood loss were 14 days, 256 minutes, 6 cm and 440 mL, respectively. Mean number of dissected nodes were 22. R0, R1 and R2 resection was achieved in 299 (91%), 15 (5%), and 13 (4%) cases, respectively with median survival of 27, 9 and 12 months in R0, R1 and R2 resections, respectively (P<0.001). Kaplan-Meier curve comparing the survival of R0 resection vs. R+ (combined R1 and R2) has been shown in Figure 4.
Comparison of various nodal dissection (Table 4) favored extensive lymphadenectomy with the best survival of 27 months after 3-FD (P<0.001).
A subgroup analysis of 32 patients, who received preoperative concurrent chemoradiation followed by surgery were found to have complete pathological response, partial response, stable disease and progression of disease in 10 (31%), 12 (38%), 9 (28%), 1 (3%), respectively. Complete responders had median survival of 25.1 vs. 12.6 months for non-responders (P=0.042).
Discussion
At diagnosis, nearly 50% of patients with esophageal cancer have cancer that extends beyond the locoregional confines of the primary. Fewer than 60% of patients with locoregional cancer can undergo a curative resection. Nearly 70% to 80% of resected specimens harbor metastases in the regional lymph nodes. Thus, clinicians are often dealing with advanced-stage carcinoma in newly diagnosed patients (7). In our series, 226 (69%) patients presented in Stage pIII with grade III dysphagia in 64.2%, supporting the above facts.
Lymph node involvement is an important prognostic indicator in esophageal carcinoma, and treatment failure is mostly related to locoregional recurrence, including the nodal recurrence. The lymphatic channels of the esophagus run vertically along the axis of the esophagus, and some of them drain into the cervical lymph glands upwards and into the abdominal glands downwards. Therefore, it is logical to conclude that not only the mediastinal lymph nodes but also the cervical and upper abdominal groups of lymph nodes are part of the regional lymphatic drainage; metastatic deposits in these nodes should not be considered as distant metastases (8). But the approach to the nodes still remains very controversial. It varies from simple sampling to two-field and three-field nodal dissection. A nationwide study in Japan showed that the rate of lymph node metastasis was 27.4% in the cervical nodes, 55.8% in the mediastinal nodes, and 43.8% in the abdominal nodes (9). The incidence of lymph node metastasis around the recurrent laryngeal nerve is between 26.7% and 48.6%. A review on the 3-FD showed the operative mortality to lie between 0% and 3.7%; similarly morbidity to vary from 37.7% to 46.7%. The rate of anastomotic leakage was 19-30% (10). Several researchers in Japan reported an excellent overall 5-year survival, varying from 30.8% to 55%. Again a nationwide study showed a better 5-year survival (34.3%) following 3-FD compared with that (26.7%) following 2-FD (P<0.001) (10). But at present there are no prospective randomized trials that compare the different approaches of lymphadenectomy in patients with locally advanced adenocarcinoma. One randomized controlled trial has compared 2-field and 3-field lymphadenectomy in squamous cell carcinoma. With 30 patients randomized to each arm, 5-year survival rates were 48% with 2-field and 66% with 3-field lymphadenectomy (not significant) (11). In our series, 280 patients (85.6%) underwent some form of radical nodal dissection with better survival results in comparison to simple nodal sampling or D1 dissection (P<0.001). The highest nodal harvest was achieved after 3-FD (40 mean nodes) and 3-FD had the best median survival result (27 months).
Besides the nodal metastasis, the other main prognostic variable is margin involvement. Some studies have shown that only 25% to 40% of patients have a resectable tumor at the time of diagnosis and only 70% of these tumors are amenable to R0 resection (12,13). We could achieve R0 resection in a substantial group of patients (91%) and this group showed significantly better survival than the R+ resection (median survival of 27 months in R0 and 9 months in R+ resection).
The long-term survival rate of patients who have undergone esophagectomy remains low. A collected review of 83,783 patients treated between 1953 and 1978 showed that the overall 5-year survival referred for surgery was only 4% (14). Another review of 43,692 patients treated between 1980 and 1988 showed only a marginal improvement in 5-year survival (4-10%) (15). In an attempt to improve the surgical results, preoperative (neoadjuvant) and postoperative (adjuvant) multimodal treatments are used.
Meta-analyses have reported an overall survival advantage with neoadjuvant chemoradiation for both adenocarcinoma and squamous cell carcinoma. An early study by Urshel and Vasan (16) included 9 randomized controlled trials with 1,116 patients, and reported a 3-year survival benefit that favored neoadjuvant chemoradiotherapy (OR 0.66; P=0.016). The survival benefit was even more pronounced when chemotherapy and radiotherapy were given concurrently (OR 0.45; P=0.005). Furthermore, patients who received neoadjuvant chemoradiation were more likely to have an R0 resection (OR 0.53; P=0.007), with 21% demonstrating complete pathologic response. The more recent meta-analysis published by Gebski and colleagues (17) evaluated 1,209 patients in 10 trials and found a survival benefit with neoadjuvant chemoradiotherapy compared with surgery alone, with a 19% decreased risk of death, corresponding to a 13% absolute difference in survival at 2 years. Another recent meta-analysis (1,854 patients, 12 randomized trials comparing preoperative chemoradiation vs. surgery alone), showed a significant survival benefit for preoperative chemoradiation in patients with resectable adenocarcinoma of the esophagus (18). Results from a recent multicenter phase III randomized trial (CROSS study), largest trial in its class, showed that preoperative chemoradiation therapy with carboplatin and paclitaxel improved overall survival compared to surgery alone in patients with resectable (T2-3, N0-1, M0) esophageal or GEJ cancers (19). Median survival was 49 months in the chemoradiation arm compared to 26 months in the surgery alone arm.
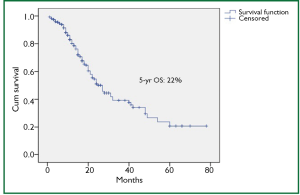
There were several limitations of our study, namely lack of use of endoscopic ultrasound (EUS) for preoperative T and N staging of the patients, only 11.6% of patients underwent neoadjuvant treatment (32 patients – preoperative concurrent CTRT and 6 patients - preoperative CT), and a retrospective design of the study. Due to the lack of EUS at our center, it was not used. As approximately 73% patients had grade III-IV dyspagia leading to gross nutritional impairment and there was a significant mean weight loss of 9.3 kg in addition to poor economical status in order to afford for perioperative total pareneteral nutrition, the usual protocol at our hospital is to take the patient directly for surgery if deemed resectable on CT films. Our previous results in 100 patients with esophageal cancer undergoing open surgery with the similar protocol had shown final pathological stages III, IVa and IVb (UICC 6th edition) in 46.6%, 4.9% and 12.6% cases, respectively (20). The overall 4-year survival for the whole group was 20%. In our present study, the 5-year overall survival was 22% with the median survival of 25 months. Only a minority of patients (9.8%) underwent preoperative chemoradiation followed by surgery and that was for radiological T4 lesions. 31% had pathological complete response in this group, which would mean a remarkable result. Besides, the complete responders had significantly higher median survival (25.1 months) than non-responders (12.6 months).
Conclusions
Our present study shows that our patients are diagnosed in advanced stage at the time of presentation. Besides stage, radical nodal dissection and R0 resection appear to be the other predictive factors for better survival. Though our post operative morbidities and mortality were in acceptable range, the overall 5 year survival of 22% needs to be improved. Preoperative chemoradiation followed by surgery appears to be a better approach than the primary surgery in our part of the world and it should become the standard practice for esophageal and GEJ cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
- Engel LS, Chow WH, Vaughan TL, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst 2003;95:1404-13.
- Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998;83:2049-53.
- Pera M, Manterola C, Vidal O, et al. Epidemiology of esophageal adenocarcinoma. J Surg Oncol 2005;92:151-9.
- Whitson BA, Groth SS, Li Z, et al. Survival of patients with distal esophageal and gastric cardia tumors: a population-based analysis of gastroesophageal junction carcinomas. J Thorac Cardiovasc Surg 2010;139:43-8.
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Mannual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96.
- Hennessy TP. The significance of three-field lymphadenectomy in oesophageal cancer. Surg Oncol 1994;3:251-3.
- Isono K, Sato H, Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology 1991;48:411-20.
- Tachibana M, Kinugasa S, Yoshimura H, et al. Extended esophagectomy with 3-field lymph node dissection for esophageal cancer. Arch Surg 2003;138:1383-9; discussion 1390.
- Nishihira T, Hirayama K, Mori S. A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg 1998;175:47-51.
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52.
- Tytgat GN, Bartelink H, Bernards R, et al. Cancer of the esophagus and gastric cardia: recent advances. Dis Esophagus 2004;17:10-26.
- Earlam R, Cunha-Melo JR. Oesophageal squamous cell carcinoma, I: A critical review of surgery. Br J Surg 1980;67:381-90.
- Mueller JM, Erasmi H, Stelzner M, et al. Surgical therapy of oesophageal carcinoma. Br J Surg 1990;77:845-57.
- Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg 2003;185:538-43.
- Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226-34.
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable esophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92.
- Gaast AV, van Hagen P, Hulshof M, et al. Effect of preoperative concurrent chemoradiotherapy on survival of patients with resectable esophageal or esophagogastric junction cancer. Results from a multicenter randomized phase III study. J Clin Oncol 2010;28:abstract 4004.
- Thakur B, Zhang CS, Meng XL, et al. Eight-year experience in esophageal cancer surgery. Indian J Cancer 2011;48:34-9.