Video-assisted thoracoscopic management for emphysema associated with contralateral destroyed lung
Introduction
Emphysema is a kind of common and disabling diseases. It is characterized by abnormal, permanent and irreversible enlargement of the air spaces distal to the terminal bronchiole and accompanied by destruction of their walls without obvious fibrosis (1). Initial medical treatments for emphysema include inhaled pharmacologic therapy with bronchodilators or corticosteroids, pulmonary rehabilitation, disease management, and supplemental oxygen.
Lung volume reduction surgery (LVRS) is a therapeutic concept that it has been successfully applied in selected patients with pulmonary emphysema (2). The procedures can be performed by median sternotomy (3), thoracotomy (4), or video-assisted thoracic surgery (VATS) (5,6). VATS has the advantage of less invasions and better recovery of postoperative pulmonary function (7-9). However, the VATS procedure can be quite challenging in condition that contralateral lung has no function (10). We presented 3 cases of emphysema associated with contralateral destroyed lung managed with the use of VATS.
Patients and methods
Case 1
A 56-year-old emphysema male suffered from a pneumothorax with persistent air leakage drained by chest tube over 1 week on the right side. Before his admission to our institution, he had experienced similar situation 2 months ago and successfully managed with the use of chest tube drainage. The CT scans showed the right pneumothorax most likely due to breakage of bullae and suggested his contralateral destroyed lung (Figure 1), which was confirmed by the patient himself later. He experienced no dyspnea before his episodes or during chest drainage. The chest tube worked well in the appropriate position and was kept for more than 3 weeks, but this time it seemed failed to resolve the situation. Therefore, VATS was performed under general endotracheal anesthesia with a double lumen endotracheal tube in December 2007. A set of cardio-pulmonary bypass apparatus stood by but without final performance. The patient was placed in the lateral decubitus position and 2 incisions were performed: one was 1.5 cm for thoracoscopy and another was 4 cm for operation. A wedge resection of the upper lobe located with bullae was performed with the use of endoscopic linear staplers. Ventilation volume was minimized and intermittent apnea was performed for the action of endoscopic staplers. Polyglycolic acid sheet (PGA) of tube type was used to reinforce the staplers for the prevention from air leak. Mechanical pleural abrasion completed the procedure. Two chest tubes were placed to water seal drainage
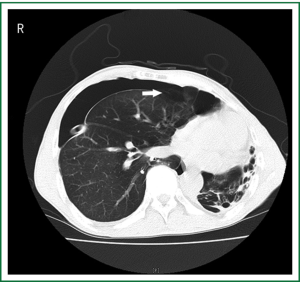
The patient was awakened and extubated in the operating room. The air leak was eventually stopped and the chest tubes were removed on day 6 and on day 16 respectively.
He was eventually discharged from the hospital on postoperative day 19. The patient experienced no recurrent pneumothorax or shortness of breath on regular follow up 18 months after surgery.
Case 2
A 57-year-old emphysema male complained of worsening dyspnea for 1 year and walking less than 20 miters. The CT scans confirmed the diagnosis of the right asymmetrical bullous lung and contralateral destroyed lung (Figure 2). His spirometry showed a forced expiratory volume in 1 second (FEV1) of 0.64 L (26% predicted) and a forced vital capacity (FVC) of 1.62 L (53% predicted). A lung volume reduction surgery (LVRS) through VATS was performed in March 2008. His incision and anesthesia was similar to the above case. Two chest tubes were also placed. The patient was mechanical ventilated and extubated on day 1 postoperatively. The two chest tubes were removed on postoperative day 3 and 5 respectively and the patient’s dyspnea was improved. He was eventually discharged from the hospital on day 15 postoperatively and experienced no major complications in the postoperative 15 months of follow-up period. His spirometry showed an improved FEV1 of 0.99 L (39.2% predicted) and a FVC of 2.14 L (68% predicted) at 7 months postoperatively. He could still walk for 1 hour and was satisfied with the life situation, which was confirmed in a telephone follow-up at 15 months postoperatively.
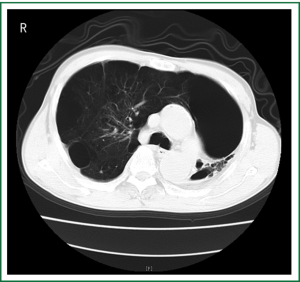
Case 3
A 47-year-old emphysema male presented with persistent air leak over 17 days due to recurrent right pneumothorax. He also experienced no dyspnea before his episodes or during chest drainage The CT scans revealed the right bullous and contralateral destroyed lungs. VATS wedge resection of the upper lobe and pleurodesis were performed similar to case 1 with 2 incisions in December 2008. The patient was extubated in the operating room. Both of his two chest tubes were removed on postoperative day 5. He was eventually discharged from the hospital on day 8 postoperatively and his right pneumothorax didn’t relapse in the postoperative 6 months of follow-up period.
Discussion
Surgeons have to face two questions when they encounter such challenging cases as above. One is whether surgery is the option, and another is how to do the surgery. Generally, the initial treatment for emphysema is medical treatments, including inhaled pharmacologic therapy with bronchodilators or corticosteroids, pulmonary rehabilitation, disease management, and supplemental oxygen. These treatments can relief the symptoms but cannot cease or reverse the development of emphysema. Surgery offers an alternative option. Emphysema patients associated with severe dyspnea, hemoptysis, recurrent pneumothorax or pneumothorax with persistent air leak, or repeated infection are suitable for surgical procedures (2). In our group of emphysema patients, case 1 and case 3 suffered from recurrent pneumothorax accompanied by persistent air leak, and case 2 suffered from worsening dyspnea despite of active medical treatment. Therefore, surgery was the consideration.
Different surgical therapies such as chest tube drainage for associated pneumothorax, lung volume reduction and lung transplantation were individually used in the selected patients (2). The therapeutic expectations in our cases were not the same. Case 1 and case 3 had no pre-operative dyspnea and their surgical indication was to solve the persistent air leak. The simplest method, chest tube drainage performed in these two cases, couldn’t resolve their problems, and bullectomy advanced treatments were needed. While in case 2, his desire was to solve the worsening dyspnea and LVRS was the option based on his lung function. In fact, the spirometry of case 2 also indicates that he was a marginal candidate for lung transplantation. However, donor shortage and his chest wall deformity due to the destroyed lung terminated the potential.
There is no existed report in the literature about surgical treatment for emphysema associated with contralateral destroyed lung. How to manage these situations with underlying contralateral dysfunctional lungs emerged as a challenge. Similar surgical conditions such as emphysema, pneumothorax and recurrent lung cancer after contralateral pneumonectomy were reported (11-16). In these reported cases, different surgical approaches such as post-lateral thoracotomies, anterior-lateral thoracotomies, muscle-sparing thoracotomies, median sternotomies or VATS were used, and the combined anaesthetic techniques included selective lobar isolation, high-frequency jet ventilation, and cardiopulmonary bypass. We chose VATS for our cases because it is the least invasive approach among the reported literatures. Theoretically, adequate operation space is necessary for VATS. General anaesthesia and single-lung ventilation are usually needed for VATS. Single-lung ventilation will collapse the lung and accommodate adequate operating space. In our cases, emphysema complicated the anaesthesia. Emphysema is the result of destructed pulmonary parenchyma with decreased mass of functioning lung tissue. It has poor elastic recoil and over-expanded volume. In addition, contralateral dysfunctional lung in our cases mandate ventilation of the remaining emphysema lung. These characters make it difficult to deflate to accommodate adequately available space particularly when it has to be mechanically ventilated. Though VATS also could be underwent under local and epidural anaesthesia, or even under local anaesthesia and sedation (17,18), VATS management for emphysema associated with contralateral destroyed lung remains a really big challenge.
Cardiopulmonary bypass is a potential alternative (19). It has the advantage to ensure thoracic surgeons the safe anesthesia and the adequate space, but the cost is more injury, procedures, complications and economic expend associated with cardiopulmonary bypass itself (20). The extra cost makes cardiopulmonary bypass not the first option and it stood by as the last defense to balance safety and mini-invisibility in our cases.
Different with the reported cases after pneumonectomy, the contralateral destroyed lung has additional risk besides its dysfunction. Destroyed lung is usually a result of inflammatory lung diseases such as tuberculosis, whole lung, necrotizing pneumonia, multiple or extensive lung abscesses, fungal infections, lung gangrene, and mycobacteria other than tuberculosis (21-27). It is a potential infection source to the operated contralateral lung. Purulent secretions from the destroyed lung could produce risks to the operated contralateral lung during anesthesia. To minimize the risk, general endotracheal anesthesia with a double lumen endotracheal tube instead of single lumen endotracheal tube is helpful. Double lumen endotracheal tube separates the contralateral lung from the secretions and facilitates cleaning it. None of our cases experienced post-operative infection on the operated lung.
Surgical intervention to the destroyed lungs or not was discussed. Destroyed lung can lead to kinds of acute life-threatening complications such as massive hemoptysis, empyema, secondary fungal infections, secondary amyloidosis, septicemia, and pulmonary-systemic shunting (22,27,28). Surgical removal of destroyed lung tissue has also been considered helpful to resolve complications and improve a patient’s quality of life. As to our cases, what they most complained was not directly associated with the destroyed lungs. The goal of their operation for this episode is to resolve the troubles resulted from emphysema. Surgical intervention to the destroyed lungs will not be considered unless urgent complications occur and mandate a pneumonectomy.
The residual lung function post operation also has to be considered. In our cases, case 2 had worst general status. He was the only person with pre-operatively worsening dyspnea and his spirometry showed poor lung function which matched the criteria for LVRS (2) and the operation was successfully completed. The other cases had better pre-operative lung function than case 2, and their medical expectations were to cease persistent air leak. The predicted amounts of lung tissues to be resected for these two cases were less than LVRS though they had no accurate spirometry due to their persistent air leak. The operation also successfully resolved their problems except that case 1 experienced post-operative air leak over 2 weeks.
In summary, emphysema with contralateral destroyed lung is a rare condition for surgical procedure. VAST can be performed with the use of general endotracheal anesthesia and a double lumen endotracheal tube was helpful to separate the contralateral lung from the secretions to reduce the risk of potential infection. To assess the condition of the patient carefully is the key to successful operation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Russi EW, De Wever W, Decramer M. Surgery for emphysema: medical aspects. In: Verleden GM, Van Raemdonck D, Leryt T, et al. eds. Surgery for non neoplastic disorders of the chest: a clinical update. European Respiratory Society monograph 2004;29:129-38.
- McKenna RJ Jr, Brenner M, Fischel RJ, et al. Should lung volume reduction for emphysema be unilateral or bilateral? J Thorac Cardiovasc Surg 1996;112:1331-8; discussion 1338-9.
- Yusen RD, Trulock EP, Pohl MS, et al. Results of lung volume reduction surgery in patients with emphysema. The Washington University Emphysema Surgery Group. Semin Thorac Cardiovasc Surg 1996;8:99-109.
- Laros CD, Gelissen HJ, Bergstein PG, et al. Bullectomy for giant bullae in emphysema. J Thorac Cardiovasc Surg 1986;91:63-70.
- Bingisser R, Zollinger A, Hauser M, et al. Bilateral volume reduction surgery for diffuse pulmonary emphysema by video-assisted thoracoscopy. J Thorac Cardiovasc Surg 1996;112:875-82.
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51.
- Kaseda S, Aoki T, Hangai N, et al. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann Thorac Surg 2000;70:1644-6.
- Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23.
- Demmy TL. Video-atlas of thoracoscopic formal lung resections emulating traditional open techniques. Ann Cardiothorac Surg 2012;1:88-99.
- Li G. Minimally invasive thoracic surgery: news from the 3rd Asian-Pacific VATS Performance & the 6th China Lung Cancer MITS Forum. J Thorac Dis 2012;4:681-7.
- Spaggiari L, Grunenwald D, Girard P, et al. Cancer resection on the residual lung after pneumonectomy for bronchogenic carcinoma. Ann Thorac Surg 1996;62:1598-602.
- Birdas TJ, Beckart DH, Keenan RJ. Contralateral spontaneous pneumothorax after pneumonectomy: thoracoscopic management with cardiopulmonary bypass. Interact Cardiovasc Thorac Surg 2005;4:27-9.
- Lewis RJ, Caccavale RJ. Pulmonary resection after pneumonectomy. Ann Thorac Surg 1997;64:583-4.
- Terzi A, Lonardoni A, Scanagatta P, et al. Lung resection for bronchogenic carcinoma after pneumonectomy: a safe and worthwhile procedure. Eur J Cardiothorac Surg 2004;25:456-9.
- Mukaida T, Andou A, Date H, et al. Thoracoscopic operation for secondary pneumothorax under local and epidural anesthesia in high-risk patients. Ann Thorac Surg 1998;65:924-6.
- Filippi AR, Mantovani C, Ricardi U. Innovative technologies in thoracic radiation therapy for lung cancer. Transl Lung Cancer Res 2012;1:263-8.
- de la Torre Bravos M, Rivas de Andrés JJ. Treatment of pneumothorax with VATS and bullectomy under local anesthesia. Video assisted thoracic surgery. Ann Thorac Surg 1999;68:2383.
- Dong Q, Liang L, Li Y, et al. Anesthesia with nontracheal intubation in thoracic surgery. J Thorac Dis 2012;4:126-30.
- Kempfert J, Rastan A, Holzhey D, et al. The learning curve associated with transapical aortic valve implantation. Ann Cardiothorac Surg 2012;1:165-71.
- Okata Y, Omura A, Yamanaka K, et al. Open reconstruction of thoracoabdominal aortic aneurysms. Ann Cardiothorac Surg 2012;1:373-80.
- Conlan AA, Lukanich JM, Shutz J, et al. Elective pneumonectomy for benign lung disease: modern-day mortality and morbidity. J Thorac Cardiovasc Surg 1995;110:1118-24
- Blyth DF. Pneumonectomy for inflammatory lung disease. Eur J Cardiothorac Surg 2000;18:429-34.
- Hammond JM, Lyddell C, Potgieter PD, et al. Severe pneumococcal pneumonia complicated by massive pulmonary gangrene. Chest 1993;104:1610-2.
- Halezeroglu S, Keles M, Uysal A, et al. Factors affecting postoperative morbidity and mortality in destroyed lung. Ann Thorac Surg 1997;64:1635-8.
- Cowles RA, Lelli JL Jr, Takayasu J, et al. Lung resection in infants and children with pulmonary infections refractory to medical therapy. J Pediatr Surg 2002;37:643-7.
- Hewitson JP, Von Oppell UO. Role of thoracic surgery for childhood tuberculosis. World J Surg 1997;21:468-74.
- Stevens MS, de Villiers SJ, Stanton JJ, et al. Pneumonectomy for severe inflammatory lung disease. Results in 64 consecutive cases. Eur J Cardiothorac Surg 1988;2
- Rizzi A, Rocco G, Robustellini M, et al. Results of surgical management of tuberculosis: experience in 206 patients undergoing operation. Ann Thorac Surg 1995;59:896-900.


