Current evidence in support of the second-generation anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor alectinib for the treatment of non-small cell lung cancer positive for ALK translocation
Treatment for advanced non-small cell lung cancer (NSCLC) depends on the molecular characteristics of the tumor. Mutations of the epidermal growth factor receptor (EGFR) gene are present in ~32% of Asians and ~7% of individuals of other ethnic groups with NSCLC (1), and rearrangements of the anaplastic lymphoma kinase (ALK) gene have been detected in ~3% to 5% of NSCLC tumors (2-4). The echinoderm microtubule-associated protein-like 4 (EML4) gene is the most common fusion partner of ALK in NSCLC, and the fusion gene exists in several variants with different breakpoints within EML4. NSCLC tumors that harbor ALK fusion genes are oncogene addicted and therefore usually sensitive to treatment with ALK tyrosine kinase inhibitors (ALK-TKIs).
Crizotinib (PF02341066), which is actually a multitarget kinase inhibitor, was the first clinically available ALK-TKI. Two pivotal phase III trials in patients with ALK translocation-positive NSCLC revealed that treatment with crizotinib conferred a significant improvement in progression-free survival (PFS) compared with cytotoxic chemotherapy in both the first- and second-line settings (5,6). Despite an initial rapid response to crizotinib treatment, however, most patients eventually develop resistance to this agent. One mechanism of such acquired resistance is a secondary mutation within the kinase domain of EML4-ALK, including a gatekeeper substitution (L1196M) similar to that (T790M) which confers resistance to EGFR-TKIs in tumors with activating mutations of EGFR (7). In addition, amplification of the ALK fusion gene as well as up-regulation of bypass signaling pathways such as those mediated by EGFR, human epidermal growth factor receptor 2 (HER2), c-KIT, or the insulin-like growth factor-1 receptor have been identified as mechanisms of crizotinib resistance (8-10).
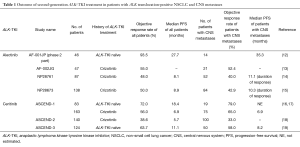
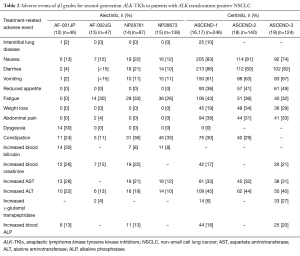
Alectinib (CH5424802) and ceritinib (LDK378) are highly selective second-generation ALK-TKIs that have been developed for the treatment of patients with NSCLC positive for ALK rearrangement. Alectinib was found to possess potent antitumor activity against ALK fusion-positive NSCLC cells that harbor the most common crizotinib resistance mutations (11). A phase 1–2 clinical trial of alectinib conducted with ALK rearrangement-positive NSCLC patients in Japan (AF-001JP study) revealed a high objective response rate (ORR) of 93.5%, a 2-year PFS rate of 76%, and a 2-year overall survival (OS) rate of 79% (12) (Table 1). A second phase 1–2 trial of alectinib, this one performed in the United States, revealed an ORR of 55% for 44 patients who progressed on, or were intolerant of, crizotinib (13). Of note, the toxicity profile of alectinib was moderate, as shown not only by most adverse events being of grade 1 or 2 but also by nausea and diarrhea of all grades being reported for only 19 (22%) and 18 (21%) patients, respectively (12) (Table 2). AF-001JP study also demonstrated that an elevation of aminotransferase levels of grade 3 or 4 was apparent in only 6% of patients, none of whom developed liver failure. On the basis of these promising results, alectinib was approved in Japan in 2014 for the treatment of metastatic or recurrent NSCLC positive for ALK translocation. In addition, the results of two phase 2 trials of alectinib for crizotinib-resistant patients with ALK translocation-positive NSCLC have recently become available. The first of these two trials, a global phase 2 study of alectinib at a dose of 600 mg twice daily, was performed with 138 enrolled crizotinib-resistant patients (15). The results revealed a high efficacy for alectinib in this group of patients, with an ORR of 50% and median PFS of 8.9 months.
Full table
Full table
The second phase 2 trial of alectinib (NP28761) was performed by Shaw et al. for patients with crizotinib-resistant NSCLC positive for ALK translocation and was recently published in Lancet Oncology (14). In this study, 87 patients (64 of whom had also received cytotoxic chemotherapy) were enrolled in the United States and Canada. Thirty-three of 69 patients with measurable disease at baseline had a confirmed partial response according to RECIST version 1.1 and as assessed by an independent review committee [ORR of 48%, with a 95% confidence interval (CI) of 36–60%]. Median PFS as estimated by Kaplan-Meier analysis was 8.1 months (95% CI, 6.2–12.6 months), a value similar to that for the previous phase 1 and 2 trials.
The toxicity profile of alectinib in the NP28761 trial was also similar to that observed in previous phase 1 and 2 studies of this agent (Table 2), with adverse events of all grades including constipation (36%), fatigue (33%), myalgia (24%), and peripheral edema (23%). The most common adverse events of grade 3 or 4 included increases in blood creatine phosphokinase (8%), alanine aminotransferase (ALT) (6%), and aspartate aminotransferase (AST) (5%). No patients developed liver failure or interstitial lung disease, providing further evidence for the tolerability of alectinib. In contrast, trials of another second-generation ALK-TKI, ceritinib, have revealed gastrointestinal side effects, including nausea and diarrhea, in ~80% of patients as well as hepatotoxicity of grade 3 or 4 in >20% of patients (16-19) (Table 2). The toxicity profile of alectinib thus compares favorably with that of other ALK inhibitors.
The new study by Shaw et al. (14) also revealed promising efficacy of alectinib for individuals with central nervous system (CNS) metastases, consistent with previous findings for both alectinib and ceritinib (Table 1). Whereas CNS metastases are manifest in 16% to 20% of all NSCLC patients at diagnosis (20,21), brain metastases have been detected in ~25% of patients with ALK rearrangement-positive NSCLC (22). The standard management for brain metastasis has been irradiation (including whole-brain radiation therapy and stereotactic radiosurgery) and surgical resection, given that traditional cytotoxic agents usually do not penetrate the blood-brain barrier. However, the possibility of systemic ALK-TKI treatment for CNS metastasis in patients with ALK translocation-positive NSCLC is receiving increasing attention. A pooled analysis of two clinical trials of crizotinib (PROFILE 1005 and 1007) revealed an intracranial objective response and disease control in 18% and 56% of patients, respectively, at 12 weeks, with a median time to progression of 7 months, in individuals with previously untreated brain metastases (23), indicative of a modest benefit of crizotinib for the treatment of such metastases. More recently, a retrospective analysis of crizotinib treatment in 59 NSCLC patients with ALK translocation, including 26 individuals with brain metastasis, revealed that the CNS was a common initial progression site and that the median PFS for patients with brain metastasis at baseline was significantly shorter than that for their counterparts without such metastasis (6.7 vs. 10.2 months, P=0.0347) (24). Crizotinib has thus shown moderate activity against intracranial disease, but the CNS has been found to be the primary site of disease progression in 50% to 70% of patients during treatment with crizotinib (5,6), suggesting that the incidence of CNS disease is increased in crizotinib-resistant cases with ALK translocation.
Penetration of alectinib into the CNS was demonstrated by analysis of paired cerebrospinal fluid and plasma samples (13). In the new study by Shaw et al. (14), 52 (60%) patients had CNS metastases at the time of enrolment, with 16 individuals—including 11 subjects previously treated with radiation therapy—having measurable CNS disease at baseline according to RECIST. Twelve of these 16 patients (75%; 95% CI, 48–93%)—including four showing a complete response—achieved an intracranial objective response, with the median duration of the CNS response being 11.1 months (95% CI, 5.8–11.1 months). In addition, of the 52 patients with measurable or nonmeasurable CNS disease at baseline, 21 (40%; 95% CI, 27–55%) achieved an objective response, including 13 (25%) with a complete response. The median duration of the CNS response in the 52 patients was also 11.1 months (95% CI, 10.8 months-not estimable), with disease control in the CNS being achieved in 46 individuals (89%; 95% CI, 77–96%).
Management of CNS metastasis in NSCLC positive for EGFR mutations may serve as a helpful reference for that of such metastasis in NSCLC positive for ALK rearrangement. A phase 2 study evaluated the first-generation EGFR-TKI gefitinib without irradiation for the treatment of brain metastases in 41 patients with EGFR mutation-positive NSCLC (25). The ORR for brain metastases, median PFS, and median OS were 87.8%, 14.5 months, and 21.9 months, respectively, suggesting that EGFR-TKIs might delay the need for irradiation and the associated risk of neurocognitive decline in such patients. More recently, osimertinib, a third-generation EGFR-TKI that is effective against the T790M gatekeeper mutant form of EGFR, was found to have efficacy in a phase 1 trial for patients with CNS metastases who had been previously treated with first- or second-generation EGFR-TKIs (26). Eight of 21 patients with CNS metastases, including those with leptomeningeal metastases, achieved a confirmed or unconfirmed response. Of note, 5 of 10 patients with a neurological disorder due to CNS metastasis showed an improvement in their neurological function. Given the similarity in the effects of EGFR mutation and ALK translocation as oncogenic driver mutations, a clinical trial of alectinib for the treatment of ALK rearrangement-positive patients with symptomatic or asymptomatic CNS metastases is warranted.
Although clinical trials of alectinib for treatment of crizotinib-resistant patients have demonstrated a durable PFS, evidence for an OS benefit in such patients is currently limited. We have reported OS data for 11 patients with ALK rearrangement-positive NSCLC treated sequentially with crizotinib and alectinib (27). The median combined PFS and OS for these patients were 18.2 and 51.1 months, respectively, suggesting that patients with ALK translocation treated with this regimen achieve durable survival. In addition, a retrospective analysis of survival in 73 ALK rearrangement-positive patients treated sequentially with crizotinib and ceritinib revealed a median combined PFS and OS of 17.4 and 49.4 months, respectively (28). Together, these previous studies suggest that sequential treatment with first- and second-generation ALK-TKIs yields a median OS of >40 months, consistent with a survival benefit of sequential therapy with crizotinib followed by a more potent ALK inhibitor after the development of crizotinib resistance in patients with NSCLC positive for ALK rearrangement.
Acknowledgements
None.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Di Lu (Nanfang Hospital, Southern Medical University, Guangzhou, China).
Conflicts of Interest: Hidetoshi Hayashi has received lecture fees from AstraZeneca K.K., Bristol Myers Squibb, and Ono Pharmaceutical Co. Ltd.; research funding from Ono Pharmaceutical Co. Ltd.; as well as advisory fees from AstraZeneca K.K. and Boehringer Ingelheim Japan Inc. Kazuhiko Nakagawa has received lecture fees and advisory fees from Chugai Pharmaceutical Co. Ltd., AstraZeneca K.K., and Nippon Boehringer Ingelheim Co. Ltd.
References
- Watanabe S, Hayashi H, Nakagawa K. Is afatinib a treatment option for brain metastases in patients with EGFR mutation-positive non-small cell lung cancer? Ann Transl Med 2016;4:225. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res 2008;14:6618-24. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734-9. [Crossref] [PubMed]
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82. [Crossref] [PubMed]
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 2012;4:120ra17. [Crossref] [PubMed]
- Tanizaki J, Okamoto I, Okabe T, et al. Activation of HER family signaling as a mechanism of acquired resistance to ALK inhibitors in EML4-ALK-positive non-small cell lung cancer. Clin Cancer Res 2012;18:6219-26. [Crossref] [PubMed]
- Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 2011;19:679-90. [Crossref] [PubMed]
- Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol 2013;14:590-8. [Crossref] [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [Crossref] [PubMed]
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234-42. [Crossref] [PubMed]
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452-63. [Crossref] [PubMed]
- Crinò L, Ahn MJ, De Marinis F, et al. Multicenter Phase II Study of Whole-Body and Intracranial Activity With Ceritinib in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer Previously Treated With Chemotherapy and Crizotinib: Results From ASCEND-2. J Clin Oncol 2016;34:2866-73. [Crossref] [PubMed]
- Felip E, Orlov S, Park K, et al. ASCEND-3: A single-arm, open-label, multicenter phase II study of ceritinib in ALKi-naïve adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:abstr 8060.
- Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002;94:2698-705. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865-72. [Crossref] [PubMed]
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108-11. [Crossref] [PubMed]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Yoshida T, Oya Y, Tanaka K, et al. Clinical impact of crizotinib on central nervous system progression in ALK-positive non-small lung cancer. Lung Cancer 2016;97:43-7. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;82:282-7. [Crossref] [PubMed]
- Yang JC, Kim DW, Kim SW, et al. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non-small cell lung cancer (NSCLC): Updated results from BLOOM, a phase I study. J Clin Oncol 2016;34:abstr 9002.
- Watanabe S, Hayashi H, Okamoto K, et al. Progression-Free and Overall Survival of Patients With ALK Rearrangement-Positive Non-Small Cell Lung Cancer Treated Sequentially With Crizotinib and Alectinib. Clin Lung Cancer 2016;17:528-34. [Crossref] [PubMed]
- Gainor JF, Tan DS, De Pas T, et al. Progression-Free and Overall Survival in ALK-Positive NSCLC Patients Treated with Sequential Crizotinib and Ceritinib. Clin Cancer Res 2015;21:2745-52. [Crossref] [PubMed]


