Phyllodes tumors of the breast: diagnosis, treatment and prognostic factors related to recurrence
Introduction
Breast tumor is still a major health burden for women, especially in urban areas (1,2), phyllodes tumor of the breast (PTB) is a rare tumor type among all kinds of breast tumor, its incidence rate is 2% to 3% in all breast fibrous epithelial tumors, or 0.3% to 1.0% in all breast tumors (3,4). In 1838, Johannes Muller first reported and named this tumor cystosarcoma phyllodes (5), described as a huge neoplasia with a cystic lobulated section and rapid growth. Over the years there have been many disputes among investigators (4) regarding the nomenclature and classification of breast phyllodes tumors. In 2003, the WHO’s international histological classification group suggested that it be named phyllodes tumor, and divided into three subtypes: benign, borderline and malignant (3). This proposal of nomenclature and classification gradually reached a consensus. The evolution of nomenclature reflects the increasing understanding of breast phyllodes tumors as a rare tumor type.
The existing breast phyllodes tumor diagnostic method has a low diagnosis accuracy in general. Preoperative diagnosis uncertainty has hindered the rational development of surgical treatment options. High local recurrence is the most important prognostic feature of this condition, with an overall recurrence rate up to 40% of all histological types of breast phyllodes tumors (6). Borderline and malignant types have a different degree of malignancy. Without adequate treatment, there will be a tendency of rapid growth and metastasis. The modes of tumor metastasis are primarily via blood, rarely lymph nodes. Common clinical sites of metastasis include the lung, followed by soft tissue, bone, pleura, etc. (7). Borderline and malignant phyllodes tumor metastasis rate is about 25% to 31%, while the overall rate of all phyllodes tumor metastasis being 4% (8,9). Improving the accuracy of diagnostic methods for breast phyllodes tumor before surgery and reducing the local recurrence rate after surgery is essential for tumor control.
Clinical and pathological characteristics
Breast phyllodes tumors occur in women aged 35 to 55 years (10). Breast phyllodes tumor lesions are often unilateral, single, nodular, painless masses with an insidious onset and slow progression. Most patients have a history of masses that grow rapidly in the short term. The tumor activity is good, free to push on the chest wall. Tumor volume varies widely, as some studies have reported that the tumor size ranges from less than 1 cm to a maximum of 40 cm in diameter (11). The relationship between tumor size and prognosis is not clear. Some studies have presented that malignant phyllodes tumor has a larger diameter compared to benign and borderline ones, while other studies have failed to reach the same conclusion (9,12). Previous studies suggest that those with a diameter of 10 cm or more is defined as a huge lobulated tumor (9). About 20% patients have palpable axillary lymph nodes in clinical examination, but only 5% are pathologically confirmed with lymph node metastasis (13). Hines et al. (14) reported that the incidence of axillary lymph node metastasis of malignant phyllodes tumor was 15%. Patients with lymph node metastasis have poor prognosis.
Breast phyllodes tumor is mostly nodular on the surface, with a clear boundary and no true capsule. It is sometimes ill-defined because some lesions invade surrounding breast tissue. The tumor section is lobulated, solid and tough, in gray or gray-yellow color. Narrow gaps or cavities of different sizes are common, containing clear or bloody fluid or jelly-like substances. The solid part is braided, with a polypoid oppressing the cysts, visible focal hemorrhage, necrosis and cystic changes. Breast phyllodes tumor includes mesenchymal and epithelial components. Benign epithelial components often form ducts or liners overlying the cavity or fracture surface. Real tumor components are hyperproliferative interstitial cells, namely fibroblasts. These cells have lost the normal arrangement, and are braided, mesh or spiral-shaped. Tumor cells can be uniformly dispersed, with unequal density in different regions and varying degrees of atypia and a variable number of mitotic figures. There may be mucoid degeneration and necrosis or hemorrhage. Epithelial components can be varying, but in general the worse the stroma differentiation, the fewer the epithelial component. Recurrent tumor histology is basically the same as primary tumor, or with a tendency to malignancy. Previous studies have shown that breast phyllodes tumor metastases contain only malignant mesenchymal components (15).
According to the diagnostic criteria established by WHO (3), benign, borderline and malignant phyllodes tumors are based on the tumor cell atypia, excessive growth, mitosis and tumor boundary (Table 1). Phyllodes tumors are sometimes observed with a fibroadenoma-like structure, where fibrocystic changes, adenosis, epithelial hyperplasia or atypical hyperplasia can occur. Invasive ductal carcinoma, lobular carcinoma and in situ carcinoma may also occur in phyllodes tumors, but they are very rare. Lobulated tumor fibroblasts can also differentiate in fat, cartilage, smooth muscle and striated muscle cells. All these components are likely to develop into a sarcoma. The presence of these components indicate poor prognosis (16).

Full table
Breast phyllodes tumor has rich biological characteristics and is difficult to predict, especially in borderline phyllodes tumor, which features two-way differentiation with obvious tendency to benign ones, clear boundary, less nuclear fission, and thus the prognosis is good. Sometimes malignant mitotic figures can be seen, and there may be bleeding, necrosis and multiple recurrence after surgery with poor prognosis. Therefore, the histological grade alone does not provide good guide for clinical treatment and prognosis. Many studies suggest that integrated histopathological prognostic indicators have more instructive significance (Table 2).

Full table
Preoperative diagnosis methods
The extensive clinical and pathological features of breast phyllodes tumors pose difficulties to the preoperative diagnosis. Imaging and cytology biopsy are the basis of preliminary judgment and classification before phyllodes tumor surgery. However, the existing breast diagnostic methods, whether it being breast ultrasound, MRI or X-ray imaging (26,27), is not characteristic of showing PTB, mostly showing the characteristics of fibroadenoma (28,29), making it difficult to distinguish them. Ultrasonography is convenient and non-invasive, so it is a preferred choice for the diagnosis of PTB (26). PTB shows on ultrasound as a bulky lobulated mass, with clear boundaries, internally mainly solid hypoechoic uneven echoes, potentially with scattered echo-free zones. Malignant phyllodes tumor does not follow the general rules of other types of breast cancer in terms of echo attenuation, and micro calcification is common. Breast X-ray findings are related to tumor size. Smaller tumors are nodules with more smooth edges, while the greater ones have more irregular lobulated yet clear borders, with higher density than normal glands. MRI can clearly show the tumor scope (27). Breast phyllodes tumor is low signal-based on plain scan T1W1, and higher signal-based on T2W1. The dynamic contrast-enhanced lesion on time-signal intensity curve is increasing more and platform type, making it easy to be differentiated with fibroadenoma. Fine needle aspiration (FNA) and core needle biopsy (CNB) is the pathology basis for preoperative diagnosis. However, due to the location and coverage limits on the amount drawn, it is difficult to differentiate with epithelial neoplasms, or other type of fibroadenoma. Therefore, the diagnostic accuracy of phyllodes tumor is low. Most studies suggest that the diagnosis accuracy rate of FNA or CNB for breast phyllodes tumor is about 50% (Table 3). For breast phyllodes tumor patients, if clinicians rely solely on the low accuracy of the preoperative examination to determine the surgical approach, or rely on pathological histological classification to determine adjuvant therapy, prognosis and tumor progress are difficult to be followed. Looking for tumor specificity for different types of phyllodes tumors with high distinction, and it is associated with tumor recurrence and metastasis of immunological markers, which is worth exploring the direction (38). Considering clinical manifestations, imaging characteristics pre-biopsy and molecular markers comprehensive intraoperative judgment can effectively improve the breast phyllodes tumor preoperative diagnosis rate. The final diagnosis and tumor histological type depends on the postoperative pathological findings.
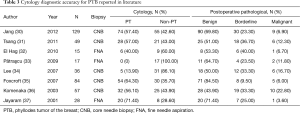
Full table
Surgical treatment
Surgery is the preferred treatment for PTB. Preoperative CNB or excision biopsy diagnosis of breast phyllodes tumor patients requires additional wide excision, surgical margins ≥1 cm. Since there are very few of these lymph node metastasis, dissection of lymph nodes is not recommended under any surgical approach. For local recurrence mass, in the absence of metastases, repeat surgery is feasible, and postoperative radiotherapy can be considered. Metastases should be treated in accordance with principles of soft tissue sarcoma. However, many problems during lobular tumors are not provided with the solution in clinical guidelines. Now there are studies supporting that breast surgical methods and surgical margin status are important factors of recurrence (Table 4). Sotheran et al. (51), Haberer et al. (52) stressed the importance of breast tumor local extended resection (WLE excision) for the control of borderline and malignant phyllodes tumor in terms of recurrence. Bhargav et al. (53) believed that regardless of how the histological grade, wide local excision should be the first choice of surgical approach, but all patients with disease recurrence were required to undergo mastectomy. Ben Hassouna et al. (24) proposed mastectomy as the preferred surgical approach for malignant phyllodes tumor. However, Kapiris et al. (49) did not find in patients with malignant phyllodes tumor the statistical significance of expanded local resection and mastectomy, and suggested the importance of negative surgical margin to control malignant phyllodes tumor recurrence and distant metastasis, consistent with the result of Moffat (50). Pandey et al. (48) found that all recurrent patients had positive surgical margins, suggesting that surgical margin was an independent risk factor for phyllodes tumor recurrence, thus improving disease-free survival (DFS) and reducing the possibility of local recurrence. Fou et al. (45) suggested that local resection of malignant phyllodes tumor circumstances to ensure negative margins can achieve a higher long-term survival. Mangi et al. (54) found that all relapse cases occurred in those with a surgical margin <1 cm. Lenhard et al. (43) also studied the surgical margins and found no difference in recurrence group and non-recurrence group. Jang et al. (30) also found no benefits in positive surgical margins more than 1 cm versus less than 1 cm in control group.
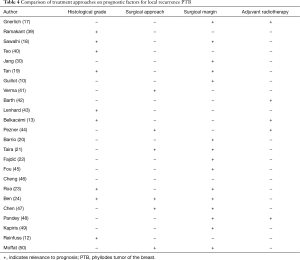
Full table
Adjuvant therapy
The efficacy of postoperative adjuvant therapy for breast phyllodes tumor is not clear. In the study of Morales-Vásquez et al. (55), there was not statistically difference between postoperative adjuvant doxorubicin and dacarbazine treatment versus no treatment in terms of survival. In the 28 malignant phyllodes tumor, 17 patients received adjuvant chemotherapy, 7 patients received postoperative radiotherapy, and the 5-year relapse-free survival was 86% in the treated group versus 58% in the untreated group (P=0.17). The exact effect of adjuvant radiotherapy for local control of different histological types of phyllodes tumor recurrence has been focused on by many investigators, but the progress is still very small. Some studies reached the same view that in the clinical management of highly malignant phyllodes tumor cases, postoperative radiotherapy can significantly reduce the likelihood of recurrence. According to the Surveillance, Epidemiology and Results Program (SEER) Program, about 50% of patients with malignant phyllodes tumors first received breast-conserving surgery, of which only fewer than 5% received postoperative adjuvant radiotherapy (56). Pezner et al. (44) indicated that for patients undergoing local resection of tumor size >2 cm and mastectomy of tumor size >10 cm, the value of adjuvant radiotherapy significantly increased. Pandey et al. (48) observed that PTB patients receiving adjuvant radiotherapy had a significantly longer 5-year DFS than those who did not receive radiation therapy (61% vs. 25%), but because of the small sample size, the study failed to show any significant difference (P=0.16) between the two groups. Belkacémi et al. (13) reported postoperative radiotherapy improved 10 years local control rate in borderline and malignant phyllodes tumor groups, but it did not affect overall survival. Other studies reported that regardless of breast-conserving surgery or mastectomy, local control rate of cases received postoperative radiotherapy group was always higher than the control group (26,57). Barth et al. (42) revealed that adjuvant radiotherapy was an effective way to control local recurrence of borderline and malignant phyllodes tumors after control breast-conserving surgery, with significantly lower relapse rate in those with negative margins receiving adjuvant radiotherapy.
Conclusions
There are different reports regarding the histological grade, surgical options, surgery margin and pathological features of breast phyllodes tumor that are closely linked with the recurrence, so we recommend to develop reasonable surgical programs based on the clinical manifestations, imaging characteristics and biopsy results, and ensure negative surgical margins; and provide necessary postoperative adjuvant therapy according to histological grade, mitotic activity, stromal cell hyperplasia and atypia.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jia M, Zheng R, Zhang S, et al. Female breast cancer incidence and mortality in 2011, China. J Thorac Dis 2015;7:1221-6. [PubMed]
- Levaggi A, Poggio F, Lambertini M. The burden of breast cancer from China to Italy. J Thorac Dis 2014;6:591-4. [PubMed]
- Tavassoli FA, Devilee P. editors. Pathology and genetics of tumours of the breast and female genital organs. World Health Organization Classification of Tumours. Lyon: International Agency for Research on Cancer (IARC) Press, 2003.
- Guerrero MA, Ballard BR, Grau AM. Malignant phyllodes tumor of the breast: review of the literature and case report of stromal overgrowth. Surg Oncol 2003;12:27-37. [Crossref] [PubMed]
- Muller J. Ueber den feinen bau unddie furmen der krankhaften geschwulste. Berlin G Reimer 1838;1:54-7.
- Parker SJ, Harries SA. Phyllodes tumours. Postgrad Med J 2001;77:428-35. [Crossref] [PubMed]
- Yagishita M, Nambu Y, Ishigaki M, et al. Pulmonary metastatic malignant phyllodes tumor showing multiple thin walled cavities. Nihon Kokyuki Gakkai Zasshi 1999;37:61-6. [PubMed]
- Khosravi-Shahi P. Management of non metastatic phyllodes tumors of the breast: review of the literature. Surg Oncol 2011;20:e143-8. [Crossref] [PubMed]
- Chaney AW, Pollack A, McNeese MD, et al. Primary treatment of cystosarcoma phyllodes of the breast. Cancer 2000;89:1502-11. [Crossref] [PubMed]
- Guillot E, Couturaud B, Reyal F, et al. Management of phyllodes breast tumors. Breast J 2011;17:129-37. [Crossref] [PubMed]
- Hawkins RE, Schofield JB, Fisher C, et al. The clinical and histologic criteria that predict metastases from cystosarcoma phyllodes. Cancer 1992;69:141-7. [Crossref] [PubMed]
- Reinfuss M, Mituś J, Duda K, et al. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer 1996;77:910-6. [Crossref] [PubMed]
- Belkacémi Y, Bousquet G, Marsiglia H, et al. Phyllodes tumor of the breast. Int J Radiat Oncol Biol Phys 2008;70:492-500. [Crossref] [PubMed]
- Hines JR, Murad TM, Beal JM. Prognostic indicators in cystosarcoma phylloides. Am J Surg 1987;153:276-80. [Crossref] [PubMed]
- Kessinger A, Foley JF, Lemon HM, et al. Metastatic cystosarcoma phyllodes: a case report and review of the literature. J Surg Oncol 1972;4:131-47. [Crossref] [PubMed]
- Barnes L, Pietruszka M. Rhabdomyosarcoma arising within a cystosarcoma phyllodes. Case report and review of the literature. Am J Surg Pathol 1978;2:423-9. [Crossref] [PubMed]
- Gnerlich JL, Williams RT, Yao K, et al. Utilization of radiotherapy for malignant phyllodes tumors: analysis of the National Cancer Data Base, 1998-2009. Ann Surg Oncol 2014;21:1222-30. [Crossref] [PubMed]
- Sawalhi S, Al-Shatti M. Phyllodes tumor of the breast: a retrospective study of the impact of histopathological factors in local recurrence and distant metastasis. Ann Saudi Med 2013;33:162-8. [PubMed]
- Tan PH, Thike AA, Tan WJ, et al. Predicting clinical behaviour of breast phyllodes tumours: a nomogram based on histological criteria and surgical margins. J Clin Pathol 2012;65:69-76. [Crossref] [PubMed]
- Barrio AV, Clark BD, Goldberg JI, et al. Clinicopathologic features and long-term outcomes of 293 phyllodes tumors of the breast. Ann Surg Oncol 2007;14:2961-70. [Crossref] [PubMed]
- Taira N, Takabatake D, Aogi K, et al. Phyllodes tumor of the breast: stromal overgrowth and histological classification are useful prognosis-predictive factors for local recurrence in patients with a positive surgical margin. Jpn J Clin Oncol 2007;37:730-6. [Crossref] [PubMed]
- Fajdić J, Gotovac N, Hrgović Z, et al. Phyllodes tumors of the breast diagnostic and therapeutic dilemmas. Onkologie 2007;30:113-8. [PubMed]
- Roa JC, Tapia O, Carrasco P, et al. Prognostic factors of phyllodes tumor of the breast. Pathol Int 2006;56:309-14. [Crossref] [PubMed]
- Ben Hassouna J, Damak T, Gamoudi A, et al. Phyllodes tumors of the breast: a case series of 106 patients. Am J Surg 2006;192:141-7. [Crossref] [PubMed]
- Asoglu O, Ugurlu MM, Blanchard K, et al. Risk factors for recurrence and death after primary surgical treatment of malignant phyllodes tumors. Ann Surg Oncol 2004;11:1011-7. [Crossref] [PubMed]
- Xu B, Hu X, Jiang Z, et al. National consensus in China on diagnosis and treatment of patients with advanced breast cancer. Transl Cancer Res 2015;4:557-73. [PubMed]
- Tallet A, Rua S, Jalaguier A, et al. Impact of preoperative magnetic resonance imaging in breast cancer patients candidates for an intraoperative partial breast irradiation. Transl Cancer Res 2015;4:148-54.
- Bode MK, Rissanen T, Apaja-Sarkkinen M. Ultrasonography and core needle biopsy in the differential diagnosis of fibroadenoma and tumor phyllodes. Acta Radiol 2007;48:708-13. [Crossref] [PubMed]
- Wurdinger S, Herzog AB, Fischer DR, et al. Differentiation of phyllodes breast tumors from fibroadenomas on MRI. AJR Am J Roentgenol 2005;185:1317-21. [Crossref] [PubMed]
- Jang JH, Choi MY, Lee SK, et al. Clinicopathologic risk factors for the local recurrence of phyllodes tumors of the breast. Ann Surg Oncol 2012;19:2612-7. [Crossref] [PubMed]
- Tsang AK, Chan SK, Lam CC, et al. Phyllodes tumours of the breast - differentiating features in core needle biopsy. Histopathology 2011;59:600-8. [Crossref] [PubMed]
- El Hag IA, Aodah A, Kollur SM, et al. Cytological clues in the distinction between phyllodes tumor and fibroadenoma. Cancer Cytopathol 2010;118:33-40. [Crossref] [PubMed]
- Pătraşcu A, Popescu CF, Pleşea IE, et al. Clinical and cytopathological aspects in phyllodes tumors of the breast. Rom J Morphol Embryol 2009;50:605-11. [PubMed]
- Lee AH, Hodi Z, Ellis IO, et al. Histological features useful in the distinction of phyllodes tumour and fibroadenoma on needle core biopsy of the breast. Histopathology 2007;51:336-44. [Crossref] [PubMed]
- Foxcroft LM, Evans EB, Porter AJ. Difficulties in the pre-operative diagnosis of phyllodes tumours of the breast: a study of 84 cases. Breast 2007;16:27-37. [Crossref] [PubMed]
- Komenaka IK, El-Tamer M, Pile-Spellman E, et al. Core needle biopsy as a diagnostic tool to differentiate phyllodes tumor from fibroadenoma. Arch Surg 2003;138:987-90. [Crossref] [PubMed]
- Jayaram G, Sthaneshwar P. Fine-needle aspiration cytology of phyllodes tumors. Diagn Cytopathol 2002;26:222-7. [Crossref] [PubMed]
- Wang Y. Development of cancer diagnostics—from biomarkers to clinical tests. Transl Cancer Res 2015;4:270-9.
- Ramakant P, Chakravarthy S, Cherian JA, et al. Challenges in management of phyllodes tumors of the breast: a retrospective analysis of 150 patients. Indian J Cancer 2013;50:345-8. [Crossref] [PubMed]
- Teo JY, Cheong CS, Wong CY. Low local recurrence rates in young Asian patients with phyllodes tumours: less is more. ANZ J Surg 2012;82:325-8. [Crossref] [PubMed]
- Verma S, Singh RK, Rai A, et al. Extent of surgery in the management of phyllodes tumor of the breast: a retrospective multicenter study from India. J Cancer Res Ther 2010;6:511-5. [Crossref] [PubMed]
- Barth RJ Jr, Wells WA, Mitchell SE, et al. A prospective, multi-institutional study of adjuvant radiotherapy after resection of malignant phyllodes tumors. Ann Surg Oncol 2009;16:2288-94. [Crossref] [PubMed]
- Lenhard MS, Kahlert S, Himsl I, et al. Phyllodes tumour of the breast: clinical follow-up of 33 cases of this rare disease. Eur J Obstet Gynecol Reprod Biol 2008;138:217-21. [Crossref] [PubMed]
- Pezner RD, Schultheiss TE, Paz IB. Malignant phyllodes tumor of the breast: local control rates with surgery alone. Int J Radiat Oncol Biol Phys 2008;71:710-3. [Crossref] [PubMed]
- Fou A, Schnabel FR, Hamele-Bena D, et al. Long-term outcomes of malignant phyllodes tumors patients: an institutional experience. Am J Surg 2006;192:492-5. [Crossref] [PubMed]
- Cheng SP, Chang YC, Liu TP, et al. Phyllodes tumor of the breast: the challenge persists. World J Surg 2006;30:1414-21. [Crossref] [PubMed]
- Chen WH, Cheng SP, Tzen CY, et al. Surgical treatment of phyllodes tumors of the breast: retrospective review of 172 cases. J Surg Oncol 2005;91:185-94. [Crossref] [PubMed]
- Pandey M, Mathew A, Abraham EK, et al. Primary sarcoma of the breast. J Surg Oncol 2004;87:121-5. [Crossref] [PubMed]
- Kapiris I, Nasiri N, A'Hern R, et al. Outcome and predictive factors of local recurrence and distant metastases following primary surgical treatment of high-grade malignant phyllodes tumours of the breast. Eur J Surg Oncol 2001;27:723-30. [Crossref] [PubMed]
- Moffat CJ, Pinder SE, Dixon AR, et al. Phyllodes tumours of the breast: a clinicopathological review of thirty-two cases. Histopathology 1995;27:205-18. [Crossref] [PubMed]
- Sotheran W, Domjan J, Jeffrey M, et al. Phyllodes tumours of the breast--a retrospective study from 1982-2000 of 50 cases in Portsmouth. Ann R Coll Surg Engl 2005;87:339-44. [Crossref] [PubMed]
- Haberer S, Laé M, Seegers V, et al. Management of malignant phyllodes tumors of the breast: the experience of the Institut Curie. Cancer Radiother 2009;13:305-12. [Crossref] [PubMed]
- Bhargav PR, Mishra A, Agarwal G, et al. Phyllodes tumour of the breast: clinicopathological analysis of recurrent vs. non-recurrent cases. Asian J Surg 2009;32:224-8. [Crossref] [PubMed]
- Mangi AA, Smith BL, Gadd MA, et al. Surgical management of phyllodes tumors. Arch Surg 1999;134:487-92; discussion 492-3. [Crossref] [PubMed]
- Morales-Vásquez F, Gonzalez-Angulo AM, Broglio K, et al. Adjuvant chemotherapy with doxorubicin and dacarbazine has no effect in recurrence-free survival of malignant phyllodes tumors of the breast. Breast J 2007;13:551-6. [Crossref] [PubMed]
- Macdonald OK, Lee CM, Tward JD, et al. Malignant phyllodes tumor of the female breast: association of primary therapy with cause-specific survival from the Surveillance, Epidemiology, and End Results (SEER) program. Cancer 2006;107:2127-33. [Crossref] [PubMed]
- EBCTCG (Early Breast Cancer Trialists' Collaborative Group), McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. [Crossref] [PubMed]


