Chest wall reconstruction after extended resection
Introduction
The first chest wall reconstruction was described by Tansini in 1906, when a pedicled latissimus dorsi flap was used to cover an anterior chest wall defect (1). Since that time, chest wall reconstruction has evolved significantly as surgical techniques have advanced and multiple prosthetic and bioprosthetic materials have become available. Chest wall defects generally result from resection of primary chest wall tumors, locally-invasive malignancies, or metastatic lesions. Direct invasion from lung or breast cancer remains the most common indication for chest wall resection (2,3). In rare instances, palliative chest wall resections are indicated to control infection, ulceration, or pain from malignancy. Regardless of the pathology being treated, extensive chest wall resection and reconstruction are challenging procedures that require careful planning and individualized treatment.
Extensive chest wall resections often result in significant morbidity and have been shown to reduce patients’ ability to perform activities of daily living (4,5). However, ongoing advances in surgical technique, postoperative care, and rehabilitation efforts have resulted in reduced rates of perioperative morbidity and mortality (6,7). The optimal approach to reconstruction of a patient’s chest wall depends on the size, location, and depth of the defect, viability of the surrounding tissue, and prior operative procedures (8). Importantly, oncologic margins should never be compromised to minimize the extent of a chest wall resection, often resulting in large, full-thickness defects (9). Therefore, a multidisciplinary approach, including input from thoracic surgeons, plastic surgeons, neurosurgeons, as well as medical and radiation oncologists is essential.
The overarching goals of all chest wall reconstructions are to obliterate dead space, restore chest wall rigidity, preserve pulmonary mechanics, protect intrathoracic organs, provide soft tissue coverage, minimize deformity, and allow patients to receive adjuvant radiotherapy, if indicated. This requires an algorithmic approach incorporating the extent and location of the resection, presence of infection, previous radiation or surgery, and other patient-specific factors such as cardiopulmonary function, lifestyle, and prognosis.
Indications for chest wall reconstruction
There are no consensus guidelines defining absolute indications for chest wall reconstruction, leading to a wide variety of surgical practices. However, most surgeons agree that defects >5 cm in diameter or including ≥4 ribs should be reconstructed due to the high risk of lung herniation and respiratory compromise from paradoxical motion of the chest wall (10-13). This is particularly true with full-thickness resections and those involving the anterolateral chest wall, where pulmonary mechanics are most affected. In contrast, some defects in the apical-posterior chest wall up to 10 cm in size do not require reconstruction because of the support provided by the scapula and shoulder girdle (14,15). An exception is when the resection extends lower than the 4th rib posteriorly, and the tip of the scapula is at risk of becoming entrapped. Even small defects in this region should be reconstructed to prevent the morbidity associated with scapular entrapment. With the increased availability of biologic materials, some surgeons have advocated for reconstruction of nearly all chest wall defects to avoid patient perception of chest wall instability or lung herniation (14).
Establishing chest wall stability
Successful chest wall reconstruction requires the re-establishment of skeletal stability. This prevents chest wall hernias, avoids thoracoplasty-like contraction of the operated side, protects underlying viscera, and maintains a cosmetically-acceptable appearance. The ideal material to re-establish chest wall stability remains controversial, however it should be malleable enough to conform to the shape of chest wall, rigid enough to prevent paradoxical motion, biologically inert, and radiolucent (16).
Each prosthetic material available for chest wall reconstruction has its own advantages and disadvantages, and none have proven to be clearly superior (15,17). The benefits of each material and technique need to be weighed against the risk of infection and other complications. Recent advances in allograft and homograft production have provided new alternatives for restoring structural stability. The decision of how to reconstruct the chest wall after extensive resection ultimately depends on the defect, surrounding tissues, cost-effectiveness, and surgeon preference.
Methyl methacrylate
Methyl methacrylate is a resin that is typically poured into a pre-shaped polypropylene (Marlex®) mesh, which is then covered with a second layer of mesh using a sandwich technique. Through an exothermic reaction, the resin becomes rigid, forming a cast that conforms to the chest wall defect. Most commonly the prosthetic is designed on the back table; however, some surgeons prefer to sew a layer of mesh in place, pour the methyl methacrylate into the defect, then cover with a second layer in situ (17). If prepared on the back table, the rigid portion of the prosthetic should be 1–2 cm smaller than the defect, which allows for a rim of mesh to be sutured to the surrounding tissues. Some have reported increased pain and atelectasis with methyl methacrylate reconstruction due to the rigid nature of the prosthesis (3,18). In addition, methyl methacrylate is not permeable to fluids, contributing to seroma formation and potentially increasing the risk of wound infection (19), however, this remains controversial (3,17). Wound complications have been reported in 10–20% of patients at 90 days, requiring removal of the prosthesis in approximately 5% of patients (3). Most agree that complete coverage with viable soft tissue is an essential step after chest wall reconstruction with methyl methacrylate to minimize the risk of local complications.
Polytetrafluoroethylene (PTFE)
PTFE (GORE-TEX®) is another material well-suited and commonly used for chest wall reconstruction. Similar to methyl methacrylate, PTFE is watertight and causes minimal foreign body reaction; however, it is flexible, allowing it to conform to the chest wall. Most commonly, 2 mm thick PTFE mesh is stretched over the chest wall defect using heavy permanent suture. To provide chest wall stability, it is important to pull the mesh as tight as possible with the sutures placed around or through adjacent ribs. A bone drill or sharp towel clip work well for creating holes in ribs for fixation. PTFE can be used to stabilize large chest wall defects and should be completely covered with viable soft tissue after implantation (Figure 1).
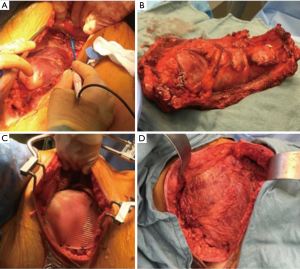
Similar to methyl methacrylate, the use of PTFE is contraindicated in infected fields. However, if the mesh becomes infected and the patient is not septic, immediate removal is not always indicated. If incision and drainage is performed, antibiotics are administered, and prosthesis removal is delayed for 6–8 weeks, enough scar tissue often forms to support the chest wall after the infected mesh is removed.
Cryopreserved homografts and allografts
Both human and porcine bioprosthetic materials have been developed over the past decade in response to the need for complex chest wall reconstruction in infected, irradiated and re-operative operative fields. Cryopreserved allografts and homografts, recovered from cadaveric donors and stored at −80 °C, are being more commonly used to restore structural integrity in cases of large chest wall defects (20,21). These materials represent a potentially limitless source of materials for chest wall reconstruction and have been shown to exhibit differences in cytotoxicity, bacterial adhesion, and biomechanical properties compared to traditional prosthetics (22). The major advantage of bioprostheses is that they are able to incorporate into native tissue with revascularization and cellular repopulation, making them more resistant to infection and useful in contaminated surgical fields (23-25). However, these materials remain quite expensive, potentially limiting their use in certain settings.
Innovative techniques using a combination of prosthetic and bioprosthetic materials, such as the “arena roof technique”, have been described for coverage of extensive chest wall defects (26). With this technique, titanium plates are anchored to the adjacent ribs, which are then covered by acellular dermal matrix, similar to a sports arena. Techniques such as these strive to adhere to the principles of biomimesis, in which anatomy is respected, function is preserved, optimal reconstructive materials are chosen, and a multidisciplinary approach to complex reconstructions is undertaken (14). Although relatively expensive, the use of titanium has several distinct advantages in chest wall reconstruction: it is corrosion-resistant and inert, has a high strength-weight ratio, can incorporate into bone, and is MRI-compatible (27). With rapid advancements in bioengineering and the popularization of 3-dimentional printing, custom-made biodegradable scaffoldings may soon become readily available.
Autologous reconstruction to provide soft tissue coverage
Regardless of the technique used to establish skeletal stability, full tissue coverage of the prosthesis is necessary. This can be achieved using direct closure, skin grafts, local advancement flaps, pedicled myocutaneous flaps, or free flaps. Therefore, plastic surgeons often play a vital role in reconstruction of extensive chest wall defects. Luckily, the thorax has a wide array of large muscle groups that can be used individually or in combination. A thorough understanding of the neurovascular anatomy of each muscle group is key to ensuring successful soft tissue transfer. Achieving full coverage becomes increasingly challenging in cases of re-operation, where many of the local muscle group flaps have already been used in previous reconstructions, or in patients with prior irradiation.
Pectoralis major flap
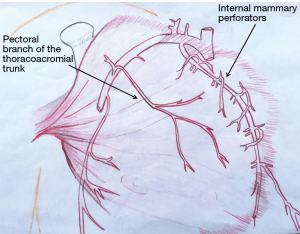
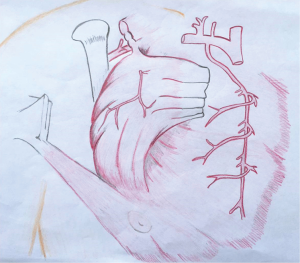
The pectoralis major muscle can be harvested and used as a muscle flap or as a myocutaneous flap with a skin paddle. It has a dual blood supply: (I) the pectoral branch of the thoracoacromial trunk of the subclavian artery; and (II) internal mammary 2nd through 6th perforatoring branches (Figure 2). The pectoralis major is primarily used as a muscle advancement or rotational flap to cover the sternum and anterosuperior chest wall defects. When pedicled off of the thoracoacromial trunk, a consistent and reliable blood supply is provided with less than 3% flap loss (28). The pectoralis can also be used as a turn-over flap, based off of the internal mammary artery perforators, which is ideal to cover midline defects, particularly if the thoracoacromial vessels have been compromised (Figure 3). Division of the humeral and clavicular insertions increases flap mobility and preserving the lateral 1/3 of the pectoralis intact will keep the anterior axillary fold intact (29). If bilateral pectoralis flaps are mobilized to cover the sternum and a future sternal resection is required, the flaps can usually be preserved and re-applied to the sternum. The pectoralis can also be passed between the superior ribs to fill apical intrathoracic dead space, when needed.
Rectus abdominus flap
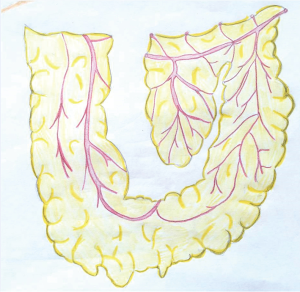
Rectus abdominus flaps are commonly used for reconstruction of chest wall defects. They can derive their blood supply from the deep inferior epigastric artery (external iliac artery) or the superior epigastric artery (subclavian artery). In addition, if the internal mammary vessels have been compromised (i.e., by prior coronary artery bypass grafting), the rectus may derive its blood supply from the 8th intercostals artery or be brought from the contralateral side (30). An overlying skin island can be transversely [transverse rectus abdominus myocutaneous (TRAM) flap] or vertically-oriented [vertical rectus abdominus myocutaneous (VRAM) flap]. Generally, VRAM skin islands have a more robust blood supply than TRAM islands due to the increased number of perforators (31). VRAM flaps are well-suited for covering large longitudinal chest wall defects, such as that left after a total sternectomy (Figure 4). TRAM flaps can cover defects up to 40 cm in size and are most often used to provide soft tissue coverage of the anterolateral chest wall. Abdominal hernias after TRAM and VRAM flaps are the most significant morbidity, being reported in up to 13% of cases (32).

Latissimus dorsi flap
The latissimus dorsi flap is the workhorse of the thoracic muscle groups used for soft tissue coverage of chest wall defects. It is based off of the thoracodorsal artery from the subscapular artery, which originates at the axillary artery (33). In the setting of a compromised thoracodorsal pedicle, the latissimus can survive off of retrograde flow from the serratus branch into the thoracodorsal artery (34). In addition, the latissimus can be used to cover midline spinal defects when based off of posterior intercostal vessel perforators. The choice of blood supply is primarily determined by the arc of rotation required to cover a given defect. Like many other muscle groups, the latissimus can be used as a muscle-only or a myocutaneous flap with a skin paddle, however the donor site must be able to be closed primarily, limiting the skin paddle to about 10 cm. Tissue expansion has been described to increase the surface area of the available skin (35). The latissimus can provide a flap up to 105 cm2 in female and 195 cm2 for males (36). It can reach anywhere on ipsilateral torso and is often used to cover anterolateral and posterior chest wall defects. Length can be gained by dissecting the muscle off of its humeral insertion or removing the pedicle from its investing fascia. Like the pectoralis, the latissimus can be passed between the ribs to fill a significant amount of intrathoracic space. This usually requires resection of a portion of 2nd or 3rd rib to avoid compression of the vascular pedicle. The large diameter of the vascular pedicle allows the latissimus dorsi to be used as a free graft, if necessary (37).
A major disadvantage of using a latissimus dorsi flap is the high rate of seroma formation, which has been reported in up to 79% of cases (38). For this reason, it is important to leave an adequate number of soft tissue drains and not remove them until the drainage is less than 25 cc per day. In addition, harvesting the latissimus leaves a large scar and results in at least temporary functional disability, with poor arm adduction between 90° and 180°. Studies have demonstrated a reduction in arm strength, particularly in women, after latissimus harvest, which commonly resolves over the first year (39,40).
Omental flap
The omentum can reach nearly any location on the chest wall and is often harvested to cover large surfaces or fill intrathoracic space. It is based off of the right or left gastroepiploic artery and contains variable amounts of fat and lymphoid tissue. The omental flap requires a laparotomy or laparoscopy and can be brought through the diaphragm or abdominal wall (41-43). It is difficult to predict the surface area of the omentum until it is visualized, since it does not correlate with patient size or obesity. Omental flaps are delicate, soft, and pliable, therefore they are most commonly used in combination with a mesh to provide stability. Graft length can be gained by mobilizing the omentum off of the greater curve of the stomach and colon and by dividing the omentum while preserving the vascular arcades (Figure 5). The primary morbidity associated with harvesting an omental flap is the risk of developing an epigastric hernia if the omentum is brought out through the abdominal wall (44,45).
External oblique flap and trapezius flap
The external oblique and trapezius flaps are less commonly employed in chest wall reconstruction, but are potential sources of soft tissue coverage for thoracic defects. The external oblique derives its blood supply from the lateral cutaneous branches of the inferior eight intercostal perforators. It can be rotated up to about the 3rd intercostal space and is most often used to cover anterolateral chest wall defects and diaphragmatic injuries. The external oblique can be harvested with a portion of the anterior rectus sheath. However, this may necessitate prosthetic reconstruction and predisposes the patient to the risk of wound complications and abdominal wall herniation.
The trapezius is a broad, diamond-shaped muscle on the posterior thorax with multiple insertions. It is can be rotated to cover upper mid-back lesions based off the transverse cervical artery and vein from the thyrocervical trunk of the subclavian vessels. A skin flap significantly larger than the muscle can be harvested with the trapezius, as long as at least 1/3 of the skin paddle overlies the muscle to ensure adequate perfusion.
Chimeric flaps
Chimeric flaps are when two or more grafts that derive their blood supply from different branches of the same vessel are harvested and used to fill or cover complex defects. The various components can be splayed out to cover large surfaces, while only requiring one vascular pedicle or anastomosis. For example, a myocutaneous flap consisting of latissimus dorsi and serratus anterior based off of the thoracodorsal vessels along with a cutaneous graft based off of the circumflex scapular vessels can be harvested with a single pedicle from the subscapular trunk. An angiogram is recommended before embarking on chimeric grafts to ensure a thorough understanding of the pertinent vascular anatomy.
Free flaps
Free flaps may be indicated if local muscle groups have been resected, previously injured, or irradiated. An extended discussion of free flaps is beyond the scope of this paper, however free flaps can play an important role in chest wall reconstruction. These grafts have been made possible with the evolution of microsurgical techniques and allow a 2-team approach to minimize operative time. The use of interposition vein grafts to bridge gaps between donor and recipient vessels has reduced pedicle length as a barrier to flap selection. The surgeon must take into account the general condition of the patient because free flaps require longer operative times. The tensor fascia lata free flap can be as large as 20×35 cm2 and is quite durable, allowing it to be sutured in place, providing stability similar to a prosthetic mesh. Harvesting the adjacent rectus femoris can add bulk to the free flap or to fill dead space. Donor-site morbidity of free flap harvest is relatively low, especially if the donor site can be closed primarily (46,47). The postoperative care of free flaps requires frequent inspections, strict positioning protocols, and may require anticoagulation.
Complications of chest wall reconstruction
Morbidity and mortality rates after chest wall resection and reconstruction range from 24–46% and 2–7%, respectively (48). These figures have been drawn over decades of experience and are likely higher than should be expected in a modern-day practice. However, wound and pulmonary complications are still most commonly observed. Wound healing complications after chest wall resection and reconstruction have been reported to be more common in patients with advanced age, multiple re-do operations, combined prosthetic and bioprosthetic reconstruction, ulcerated tumors, and in those who have had soft tissue coverage with omentum (25,49). However, wound vacuum coverage has been suggested to reduce the need for prosthesis removal and may help achieve healing in patients with postoperative wound infections (25).
Likewise, resection of portions of the chest wall and musculature poses a formidable risk of developing postoperative pulmonary complications. This risk is likely heightened when en bloc pulmonary resection is performed (4). After propensity matching 34 patients who underwent anatomic lung resection via thoracotomy to 34 who underwent anatomic lung resection with en bloc chest wall resection, we recently examined the relationship between number of ribs resected and postoperative respiratory complications. On multivariate analysis, having 1–3 ribs resected (OR, 19.29; 95% CI, 1.33–280.72; P=0.03), 4–6 ribs resected (OR, 26.66; 95% CI, 1.48–481.86; P=0.03), and a lower DLCO (OR, 0.91; 95% CI, 0.84–0.99; P=0.02) were independently associated with postoperative respiratory complications (bronchoscopy, re-intubation, pneumonia, or tracheostomy) (4). It should be recognized, however, that without chest wall reconstruction, the incidence of flail chest and paradoxical motion, and resultant pulmonary complications, would likely be higher than with reconstruction.
Conclusions
Reconstruction of extensive chest wall defects following chest wall resection can be a formidable challenge. This can be best achieved by adhering to the principles of biomimesis, in which anatomy is respected, function is preserved, optimal reconstructive materials are chosen, and a multidisciplinary approach to complex reconstructions is undertaken. First skeletal stability must be established with prosthetic or bioprosthetic materials, or a combination of both. It is then imperative that soft tissue coverage be achieved, using one of multiple available rotational, advancement, or free flaps. This requires a precise understanding of the neurovascular anatomy of each muscle group to ensuring successful soft tissue transfer. With the rapid evolution of new materials, such as custom biodegradable scaffoldings, and innovation in surgical techniques, outcomes for extensive chest wall resection and reconstruction are expected to continue to improve.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tansini I. Sopra il mio nuovo processo di amputazione della mammella. Gazzetta Med Ital 1906;57:141-2.
- Mansour KA, Thourani VH, Losken A, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg 2002;73:1720-5; discussion 1725-6.
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85. [Crossref] [PubMed]
- Geissen NM, Medairos R, Davila E, et al. Number of Ribs Resected is Associated with Respiratory Complications Following Lobectomy with en bloc Chest Wall Resection. Lung 2016;194:619-24. [Crossref] [PubMed]
- Daigeler A, Druecke D, Hakimi M, et al. Reconstruction of the thoracic wall-long-term follow-up including pulmonary function tests. Langenbecks Arch Surg 2009;394:705-15. [Crossref] [PubMed]
- Tukiainen E, Popov P, Asko-Seljavaara S. Microvascular reconstructions of full-thickness oncological chest wall defects. Ann Surg 2003;238:794-801; discussion 801-2. [Crossref] [PubMed]
- van Geel AN, Wouters MW, Lans TE, et al. Chest wall resection for adult soft tissue sarcomas and chondrosarcomas: analysis of prognostic factors. World J Surg 2011;35:63-9. [Crossref] [PubMed]
- Ferraro P, Cugno S, Liberman M, et al. Principles of chest wall resection and reconstruction. Thorac Surg Clin 2010;20:465-73. [Crossref] [PubMed]
- McAfee MK, Pairolero PC, Bergstralh EJ, et al. Chondrosarcoma of the chest wall: factors affecting survival. Ann Thorac Surg 1985;40:535-41. [Crossref] [PubMed]
- Netscher DT, Baumholtz MA. Chest reconstruction: I. Anterior and anterolateral chest wall and wounds affecting respiratory function. Plast Reconstr Surg 2009;124:240e-52e. [Crossref] [PubMed]
- Mahabir RC, Butler CE. Stabilization of the chest wall: autologous and alloplastic reconstructions. Semin Plast Surg 2011;25:34-42. [Crossref] [PubMed]
- Losken A, Thourani VH, Carlson GW, et al. A reconstructive algorithm for plastic surgery following extensive chest wall resection. Br J Plast Surg 2004;57:295-302. [Crossref] [PubMed]
- Arnold PG, Pairolero PC. Chest-wall reconstruction: an account of 500 consecutive patients. Plast Reconstr Surg 1996;98:804-10. [Crossref] [PubMed]
- Rocco G. Chest wall resection and reconstruction according to the principles of biomimesis. Semin Thorac Cardiovasc Surg 2011;23:307-13. [Crossref] [PubMed]
- Deschamps C, Tirnaksiz BM, Darbandi R, et al. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg 1999;117:588-91; discussion 591-2. [Crossref] [PubMed]
- le Roux BT, Shama DM. Resection of tumors of the chest wall. Curr Probl Surg 1983;20:345-86. [Crossref] [PubMed]
- Lardinois D, Müller M, Furrer M, et al. Functional assessment of chest wall integrity after methylmethacrylate reconstruction. Ann Thorac Surg 2000;69:919-23. [Crossref] [PubMed]
- Aghajanzadeh M, Alavy A, Taskindost M, et al. Results of chest wall resection and reconstruction in 162 patients with benign and malignant chest wall disease. J Thorac Dis 2010;2:81-5. [PubMed]
- Chapelier AR, Missana MC, Couturaud B, et al. Sternal resection and reconstruction for primary malignant tumors. Ann Thorac Surg 2004;77:1001-6; discussion 1006-7. [Crossref] [PubMed]
- Marulli G, Hamad AM, Cogliati E, et al. Allograft sternochondral replacement after resection of large sternal chondrosarcoma. J Thorac Cardiovasc Surg 2010;139:e69-70. [Crossref] [PubMed]
- Rocco G, Fazioli F. Cryopreserved biomaterials for chest wall reconstruction. Multimed Man Cardiothorac Surg 2009;2009:mmcts.2008.003277.
- Wiegmann B, Korossis S, Burgwitz K, et al. In vitro comparison of biological and synthetic materials for skeletal chest wall reconstruction. Ann Thorac Surg 2015;99:991-8. [Crossref] [PubMed]
- Rocco G, Fazioli F, Scognamiglio F, et al. The combination of multiple materials in the creation of an artificial anterior chest cage after extensive demolition for recurrent chondrosarcoma. J Thorac Cardiovasc Surg 2007;133:1112-4. [Crossref] [PubMed]
- Judas F, Rosa S, Teixeira L, et al. Chondrocyte viability in fresh and frozen large human osteochondral allografts: effect of cryoprotective agents. Transplant Proc 2007;39:2531-4. [Crossref] [PubMed]
- Rocco G, Martucci N, La Rocca A, et al. Postoperative local morbidity and the use of vacuum-assisted closure after complex chest wall reconstructions with new and conventional materials. Ann Thorac Surg 2014;98:291-6. [Crossref] [PubMed]
- Rocco G, La Rocca A, La Manna C, et al. Arena Roof Technique for Complex Reconstruction After Extensive Chest Wall Resection. Ann Thorac Surg 2015;100:1479-81. [Crossref] [PubMed]
- Thomas PA, Brouchet L. Prosthetic reconstruction of the chest wall. Thorac Surg Clin 2010;20:551-8. [Crossref] [PubMed]
- Daigeler A, Falkenstein A, Pennekamp W, et al. Sternal osteomyelitis: long-term results after pectoralis muscle flap reconstruction. Plast Reconstr Surg 2009;123:910-7. [Crossref] [PubMed]
- Nahai F, Morales L Jr, Bone DK, et al. Pectoralis major muscle turnover flaps for closure of the infected sternotomy wound with preservation of form and function. Plast Reconstr Surg 1982;70:471-4. [Crossref] [PubMed]
- Nguyen DT, Aoki M, Hyakusoku H, et al. Chest wall reconstruction of severe mediastinitis with intercostal artery-based pedicled vertical rectus abdominis muscle flap with oblique-designed skin pedicle. Ann Plast Surg 2011;67:269-71. [Crossref] [PubMed]
- Villa MT, Chang DW. Muscle and omental flaps for chest wall reconstruction. Thorac Surg Clin 2010;20:543-50. [Crossref] [PubMed]
- Daigeler A, Simidjiiska-Belyaeva M, Drücke D, et al. The versatility of the pedicled vertical rectus abdominis myocutaneous flap in oncologic patients. Langenbecks Arch Surg 2011;396:1271-9. [Crossref] [PubMed]
- Rowsell AR, Eisenberg N, Davies DM, et al. The anatomy of the thoracodorsal artery within the latissimus dorsi muscle. Br J Plast Surg 1986;39:206-9. [Crossref] [PubMed]
- Fisher J, Bostwick J 3rd, Powell RW. Latissimus dorsi blood supply after thoracodorsal vessel division: the serratus collateral. Plast Reconstr Surg 1983;72:502-11. [Crossref] [PubMed]
- Slavin SA. Improving the latissimus dorsi myocutaneous flap with tissue expansion. Plast Reconstr Surg 1994;93:811-24. [Crossref] [PubMed]
- Serafin D. The latissimus dorsi muscle—musculocutaneous flap. In: Serafin D. editor. Atlas of microsurgical composite tissue transplantation. Philadelphia: WB Saunders, 1996:208.
- Sauerbier M, Dittler S, Kreutzer C. Microsurgical chest wall reconstruction after oncologic resections. Semin Plast Surg 2011;25:60-9. [Crossref] [PubMed]
- Menke H, Erkens M, Olbrisch RR. Evolving concepts in breast reconstruction with latissimus dorsi flaps: results and follow-up of 121 consecutive patients. Ann Plast Surg 2001;47:107-14. [Crossref] [PubMed]
- Fraulin FO, Louie G, Zorrilla L, et al. Functional evaluation of the shoulder following latissimus dorsi muscle transfer. Ann Plast Surg 1995;35:349-55. [Crossref] [PubMed]
- Glassey N, Perks GB, McCulley SJ. A prospective assessment of shoulder morbidity and recovery time scales following latissimus dorsi breast reconstruction. Plast Reconstr Surg 2008;122:1334-40. [Crossref] [PubMed]
- Romanini MV, Vidal C, Godoy J, et al. Laparoscopically harvested omental flap for breast reconstruction in Poland syndrome. J Plast Reconstr Aesthet Surg 2013;66:e303-9. [Crossref] [PubMed]
- Acarturk TO, Swartz WM, Luketich J, et al. Laparoscopically harvested omental flap for chest wall and intrathoracic reconstruction. Ann Plast Surg 2004;53:210-6. [Crossref] [PubMed]
- Saltz R, Stowers R, Smith M, et al. Laparoscopically harvested omental free flap to cover a large soft tissue defect. Ann Surg 1993;217:542-6; discussion 546-7. [Crossref] [PubMed]
- Hultman CS, Culbertson JH, Jones GE, et al. Thoracic reconstruction with the omentum: indications, complications, and results. Ann Plast Surg 2001;46:242-9. [Crossref] [PubMed]
- Jurkiewicz MJ, Arnold PG. The omentum: an account of its use in the reconstruction of the chest wall. Ann Surg 1977;185:548-54. [Crossref] [PubMed]
- Fischer S, Klinkenberg M, Behr B, et al. Comparison of donor-site morbidity and satisfaction between anterolateral thigh and parascapular free flaps in the same patient. J Reconstr Microsurg 2013;29:537-44. [Crossref] [PubMed]
- Klinkenberg M, Fischer S, Kremer T, et al. Comparison of anterolateral thigh, lateral arm, and parascapular free flaps with regard to donor-site morbidity and aesthetic and functional outcomes. Plast Reconstr Surg 2013;131:293-302. [Crossref] [PubMed]
- Bennett DT, Weyant MJ. Extended chest wall resection and reconstruction in the setting of lung cancer. Thorac Surg Clin 2014;24:383-90. [Crossref] [PubMed]
- Lans TE, van der Pol C, Wouters MW, et al. Complications in wound healing after chest wall resection in cancer patients; a multivariate analysis of 220 patients. J Thorac Oncol 2009;4:639-43. [Crossref] [PubMed]


