Tracheobronchial tumors
Introduction
Pure tracheobronchial tumors (TBTs) are a rare entity that may present with diverse pathological findings and may challenge in their diagnosis and management. The malignant tumors are more frequent than benign ones. Although TBT tumors represent only 0.6% of all pulmonary tumors, they are clinically significant (1,2). These tumors are often revealed incidentally.
The symptoms of an endobronchial tumor primarily depend on its size rather than pathologic characteristics of the tumor itself. They may be clinically significant, causing airway obstruction and mimicking malignant neoplasms and asthma or chronic obstructive pulmonary disease (COPD) (1-3). Today, novel radiological techniques and better access to flexible bronchoscopy enable detection of larger number of TBT. We present a review of tracheal and bronchial tumors and discuss significant aspects of the different TBT with focus on clinical manifestations and diagnostic procedures.
Diagnosis
In the early stages, both benign and malignant endobronchial tumors may have similar signs and symptoms which can be misdiagnosed as an asthma, COPD or pulmonary infection. Most commonly, the patients seek treatment because of cough and hemoptysis, chest pain, dyspnea, localized wheezing, recurrent pneumonia, or atelectasis due to bronchial obstruction. In the absence of airway obstruction, the patient may be asymptomatic (1-7). Lung function may be impaired in patients with TBT. Maximal voluntary ventilation and
The body plethysmography and impulse oscillometry are useful in detecting increased respiratory resistance in patients with tracheal and bronchial tumors (11).
Delays in diagnosis of tracheal or endobronchial tumors commonly occur because the signs and symptoms caused by these tumors are nonspecific and chest radiographs are often considered unremarkable. The chest radiographic may demonstrate normal findings, a solitary pulmonary nodule, or bronchial obstruction with distal atelectasis or consolidation. Therefore, if there is clinical or radiographic suspicion of a TBT, further evaluation with computed tomography (CT) is recommended (5,12,13). CT imaging is the standard imaging tool for diagnosis and evaluation of tumor extent. It may demonstrate a tracheal or bronchial mass and/or associated findings of distal bronchial dilatation with mucoid impaction, post-obstructive pneumonia, subsegmental atelectasis or air trapping, indicating the endobronchial location of the tumors. More important, CT findings can exclude contiguous mediastinal and parenchymal lung involvement (12,13).
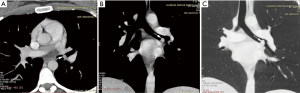
The diagnosis of TBTs has been significantly improved by recent advances such as the routine use of multi-detector CT (MDCT) with thin section reconstructions and techniques such as 2D minimum-intensity-projection and post-processing techniques like multi-planar reformation (MPR), volume rendering (VR) and virtual bronchoscopy (VB). The combination of these techniques could help to reveal tumor locations and morphologies, extramural invasions of tumors, longitudinal involvements of tumors, morphologies and extents of luminal stenosis, distances between main bronchus tumors and tracheal carina, and internal features of tumors (Figure 2) (14,15). VB can show a real-like anatomical view of the tracheobronchial tree. Its images are in a similar fashion to conventional optical bronchoscopy. But, VB has some disadvantages, because it lacks the detail of real bronchoscopy and cannot show mucosal or submucosal extensions. However, VB is superior in bypassing any obstruction and therefore providing an excellent view distal to the high grade stenosis. This imaging modality provides information that may be beneficial in the management of patients with TBT (15-18). Magnetic resonance imaging (MRI) may have a role in individual patients, either when CT is unavailable or when radiation must be avoided in patients who need repeated imaging. It has been reported to be useful in adenoid cystic carcinoma (ACC) and fat containing lesions, as a lipoma and hamartoma (12). Endoluminal ultrasound may be used for evaluating the extent of airway tumor and its relation to the adjacent structure. It is possible to distinguish compression from infiltration of the airway by an extrinsic tumor, but detection of submucosal infiltration in the presence of a normal mucosal surface has so far not been described (19,20).
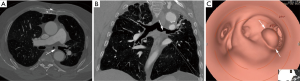
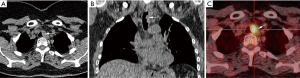
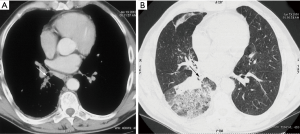
Another diagnostic tool that is increasingly being used in the diagnosis of various tumors that are suspected of being malignant is fluorine 18 fluorodeoxyglucose (FDG) positron emission tomography (PET). Increased FDG PET/CT uptake at the obstruction site is very suggestive of malignancy, while benign lesions show low FDG uptake (Figure 3) (21,22). Bronchoscopy is the main diagnostic tool for TBT because it allows direct visualization of tumor, tissue sampling and disease staging. Also, bronchoscopy reveals the localization, extent of TBT, as its relation to the surrounding structure (23).

The pulmonologist, thoracic surgeon and anesthesiologist should make multidisciplinary team and make the decision for TBT treatment. Good treatment results should be obtained using carefully planned and well-executed therapeutic approach. Therapeutic bronchoscopy with different interventional procedures (tumor resection, electrocautery, cryotherapy, argon plasma coagulation, laser, i.e.,) should open the blocked airways. Surgical treatment (sleeve resection, lobectomy, pneumonectomy) should be performed to obtain negative surgical margins while sparing lung parenchyma (7). The prognosis of TBTs is variable and depends on multiple factors, as location, malignant potential, co-morbidities, lymph node involvement and invasion of mediastinal vital organs.
TBTs are rare in children, more often malignant than benign, with wide array of pathologic findings. In childhood, TBT presents with respiratory symptoms not improving with antibiotics and bronchodilators. Some patients have symptoms of recurrent pneumonia. Carcinoid tumors usually manifest as cough and recurrent wheezing. The wheezing is thought to be attributable in part to the obstruction but also to serotonin release by the tumor in the respiratory tract (24,25).
Characteristics of specific TBTs
Tumors in the tracheobronchial tree can be classified as primary malignant tumors, secondary malignant tumors, or benign tumors. Primary malignant tumors commonly originate from surface epithelium or the salivary glands, while benign tumors from mesenchymal tissue (Table 1) (6).
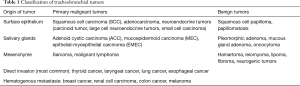
Full table
Malignant tumors
Most tumors of the tracheobronchial tree are malignant, whereas benign tumors are very rare. The predominant types of malignant TBT are squamous cell carcinoma (SCC), small cell carcinoma, carcinoid tumor and mucoepidermoid carcinoma (MEC). SCC and small cell carcinoma are most common types of primary lung carcinoma which originate from the large bronchi. Both tumors are highly associated with cigarette smoking. Symptoms are related to the degree of bronchial obstruction an include cough, hemoptysis. Secondary malignant tumors in the airways are uncommon and may occur as a result of hematogenous spread or direct invasion by a malignancy of an adjacent organ. Airway invasion by primary neoplasms arising from adjacent organs is more frequent than hematogenous metastases. Local tracheal invasion from adjacent cancers of the esophagus, thyroid, or lung is more common than hematogenous spread to the mucosa (4,21).
Primary malignant tumors
SCC
Primary tracheal neoplasm is very rare. Reported frequencies range from 0.075% in autopsy series to 0.19% of all patients with malignancies of the respiratory tract. Ninety percent of all adult primary tracheal neoplasms are malignant with common histopathological patterns comprising of SCC, ACC, carcinoid, MEC, and papilloma (13). SCC are aggressive neoplasms associated with smoking, with a peak incidence between the ages of 50 and 70 years (12,14). SCC is the most common tracheal tumor and is two to four times more common in men. It is histologically identical to lung SCC. SCC is commonly asymptomatic. Symptoms usually appear when the tumor occludes more than 50% of the airway diameter and include cough, hoarseness, dyspnea, hemoptysis, and wheeze (6,26-28). At CT, the tumor may appear as a polypoid lesion, a focal sessile lesion, eccentric narrowing of the airway lumen or circumferential wall thickening generally in the lower third of trachea. This tumor typically has irregular margins as it arises from the surface epithelium. It may invade mediastinum by direct extension or lymphatic spread (12,13,15).The majority of SCCs of the tracheobronchial tree show high uptake at FDG PET (Figure 3C) (21).
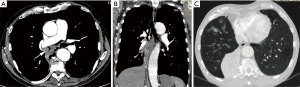
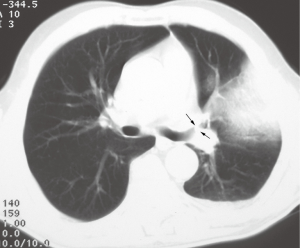
Endobronchial SCC causes airway obstruction by mass itself and leads to pulmonary atelectasis or lobar collapse. SCC is the most common cell type which cavitate, in approximately 10% of cases (16). At CT, endoluminal mass has the same appearance as in trachea (Figure 4). The primary mass may be differentiated from atelectatic lung and mucus regarding the different contrast enhancement or by different signal intensity on MRI. Small cell cancer is the second most common lung cancer arising in the central bronchi. Bronchial obstruction is much less common with small cell carcinoma than with SCC. The most common imaging finding in small cell carcinoma is extensive hilar or mediastinal lymphadenopathy secondary to early metastasis (13,16).
Carcinoid tumors
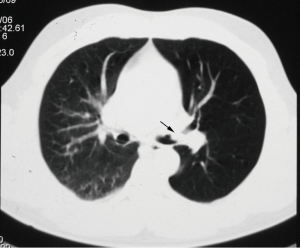
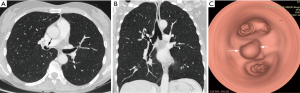
Bronchial carcinoid tumors account for over 25% of all carcinoid tumors and for 1–2% of all pulmonary neoplasms. Typical pulmonary carcinoid tumors are more frequent with approximately 80–90% of cases (12). It usually involves the main, lobar, or segmental bronchi. Bronchial carcinoids range from low-grade typical carcinoids to intermediate-grade atypical carcinoids to high-grade small cell carcinomas and demonstrate a wide spectrum of clinical manifestations and histologic features. Bronchial carcinoids affect male and female patients equally with a mean patient age of 45 years (2,29-31). Patients with atypical carcinoid are significantly older than those with typical carcinoid while patients with bronchial carcinoids are younger than those with bronchogenic carcinoma (32). Large series showed that smoking is associated with atypical but not with typical carcinoids (31). Most series reported about 25% of patients without symptoms, so that bronchial carcinoids are an incidental finding (32). Most of symptoms and signs are the consequence of bronchial obstruction and include dyspnea, cough, recurrent pulmonary infection, fever, expectoration, wheezing, hemoptysis, and chest pain. Some patients are misdiagnosed as asthma. In series of Filosso et al. a total of 14.2% of patients had been treated for asthma for up to 3 years before the tumor was discovered (31). Hemoptysis occurs in at least 50% of patients, reflecting the rich vascularization of these neoplasms. Paraneoplastic syndromes are rare and include carcinoid syndrome, Cushing’s syndrome, and ectopic growth hormone-releasing hormone secretion (12,30,31). Because of the central location of carcinoids, initial chest-ray may demonstrate normal finding or findings related to bronchial obstruction like atelectasis, obstructive pneumonitis or air trapping (Figure 5). Central bronchial carcinoids most common are manifested as a hilar or perihilar mass (2,29,32,33). The mass is usually a sharply bordered, round or ovoid lesion and may be slightly lobulated at radiography and CT. On CT, endobronchial carcinoid tumor appears as a well-defined spherical or oval mass with a slightly lobulated border and frequently intensely enhanced (Figure 6). However, not all carcinoids show contrast enhancement but enhancement alone does not allow differentiation of bronchial carcinoid from bronchogenic carcinoma (33-35). Extraluminal component of the tumor which is not visible bronchoscopically, could be presented at CT. Furthermore, typical carcinoids are smaller than atypical carcinoids which may have irregular contours and less uniform contrast enhancement (36). Peripheral carcinoids can manifested as nodules, non-segmental infiltrates or pleural effusion. In reported series nodules were most common manifestation of peripheral carcinoid tumors (2,34). The eccentric calcifications, especially in central carcinoids, are usually not identified at conventional radiography but are clearly visible at CT in up to 26% (12). It has been reported that an ultrafast contrast-enhanced magnetic resonance (MR) imaging shows a pronounced and rapid increase in signal intensity in bronchial carcinoids (12,13). Both typical and atypical carcinoids may be associated with hilar or mediastinal lymphadenopathy due to hyperplasia from recurrent pneumonia or metastasis (2,37). Most studies have been reported that carcinoid tumors may show increased FDG uptake due to high metabolic activity and malignant potential but still lower than would be expected for malignant tumors (21,22). Scintigraphy with 111In-octreotide has shown valid uptake in primary tumors and the ability to detect early recurrences and metastases even in asymptomatic patients which might be a useful tool for the staging in the future (31). Since most bronchial carcinoids are in a central location they are within reach of a bronchoscope. These tumors have a characteristic bronchoscopic appearance as smooth, cherry red, polypoid endobronchial nodules (2).
Salivary glands malignant tumors
Primary salivary gland-type tumors of the lung are rare, accounting for fewer than 2% of all lung cancers. These tumors characteristically grow intraluminally in the central airway, tend to occur in non-smokers and younger patients and have a much better prognosis than the more common types of lung cancer (adenocarcinoma and SCC). The most common histologic type is ACC, comprising about two thirds of the cases, whereas MEC comprises about a third and only a few EMEC primary salivary gland-type tumors of the lung have been described in the literature (20,38,39).
ACC
ACC is the most common salivary-type tumor of large airways and the second common primary tracheal tumor with a prevalence of 0.04–0.2% (19). It should be considered as differential diagnosis, especially in younger adults with tumors in trachea and/or large bronchi (40-42). Most studies reported female predominance and no association with smoking (43). Since the longitudinal extent is typically greater than the cross-sectional area it may even involve the entire trachea and extend into the main bronchi. It also arises in main stem or lobar bronchi without tracheal involvement (13,44). Symptoms in patients with ACC are non-specific and some studies divided them in two groups: those related to airway obstruction and symptoms associated with lung infections. Dyspnea, cough, stridor, wheezing, and hemoptysis are the most common complaints. Unfortunately, these signs and symptoms often lead to a misdiagnosis as asthma or chronic bronchitis which delays in reaching a definitive diagnosis. Therefore, most patients present with locally advanced disease. But, despite this fact, prognoses are good unless metastases are detected (45).
The CT scan is a useful imaging procedure for ACC. It is highly accurate in the assessment of the tumor location, extra luminal extensions, carinal involvement and distant metastasis. At CT, ACC appears as a focal mass in the trachea or main bronchi with a smooth border as it arises from submucosa. It may involve more than half of the airway circumference. This tumor can also be differentiated from MEC because of its frequent extra-luminal extension. Lymphadenopathy and distant metastases are uncommon and the local recurrence is most common (13).
The largest imaging studies reported that FDG uptake in primary salivary gland-type tumors of the lung is variable, depending on the grade of differentiation; like in other indolent tumors (39,43). Timely diagnosis of ACC is important because prognosis is improved with early treatment (21,22).
MEC
MEC of tracheobronchial tree is rare-occurring tumor, comprising only 0.1–0.2% of all lung malignancies and affecting equally males and females at median age of 40 years (from 3 to 78 years), not correlated with smoking. It arises from minor salivary glands lining the tracheobronchial tree and originates more common in the lobar bronchi than in the trachea or main bronchi. Fewer than 15% of cases occur in the lower trachea and rarely tumor presents as an isolated peripheral pulmonary nodule. On the basis of histologic criteria, this tumor is classified as either low grade or high grade (46,47). Several studies (43,47) reported that patients with low grade MEC are younger than those with high grade tumor. Symptoms of MEC are nonspecific and depend on the degree of bronchial obstruction. Cough, hemoptysis, pneumonia and fever were reported as most common clinical symptoms of MEC, although one third of patients can be asymptomatic (47). Radiographic features of MEC are related to bronchial obstruction. In some cases, solitary peripheral nodule can be seen. CT enable visualization of endoluminal tumor mass, even when the tumor is located within a segmental bronchus. It appears as a non-spherical, smooth, lobulated, mild enhanced intraluminal mass adapting to the branching features of the lobar bronchus. It is often associated with dilated distal bronchi, mucoid impaction and distal atelectasis. Peripheral nodules appear as a smooth round nodule less than 2 cm in size. Punctate calcifications within the tumor are seen in half of the patients. Metastases to the regional lymph node are rare, and the prognosis is excellent (13,39,48). Reported series showed variable amounts and patterns of FDG uptake in MEC resembling bronchial carcinoid. In doubtful cases additional 68Ga-DOTATOC PET CT could be performed. Positive finding suggest typical carcinoid, and negative MEC. MECs in the central airways with high FDG uptake may be difficult to distinguish from bronchogenic carcinoma (12,21,49).
At bronchoscopy, MECs of the trachea or bronchi usually appear as exophytic intraluminal, pink, highly vascularized mass which can be sessile polypoid with a broad base connected to the bronchial wall or pedunculated with a well-formed stalk. This finding may resemble carcinoid tumor (50).
Metastases
Metastatic involvement of the trachea and large airways is rare and may occur as a result of hematogenous spread or direct invasion by a malignancy of the lung, larynx, esophagus, thyroid gland, or mediastinum. The most common primary tumors that spread hematogenously to the large airways are breast, colon, kidney, lung and melanoma (51). At CT, these tumors can present as solitary or multiple polypoid nodules (Figure 7) with or without the “glove-finger” appearance, or as eccentric wall thickening (21). In rare cases when the metastasis is solitary, it is indistinguishable from a primary airway tumor (i.e., SCC, ACC, or carcinoid).
In cases of direct airway spread from a primary tumor, the important clue to the diagnosis is to establish that the tumor is centered outside the airway, for example, in the esophagus, lung, or thyroid. A history of primary adenocarcinoma or melanoma suggests metastatic involvement of the airways. In most cases, the extramural source of an airway tumor is apparent at CT (13,52).
The degree of FDG uptake depends mostly on the metabolic activity and degree of differentiation of the primary tumor. Since the majority of malignancies have high metabolic activity, FDG PET usually shows intense uptake (12,21).
Benign endobronchial tumors
Benign tumors of the tracheobronchial tree are quite rare and constitute 2% of all lung tumors. Histologically, the most frequent benign TBTs are hamartomas and papillomas. Less frequent benign neoplasms are leiomyomas, lipomas, chondromas, and neurogenic tumors. These tumors are slow growing and usual presentation is related to bronchial obstruction. Benign lesions of the trachea often remain undiagnosed for months or even years, misdiagnosed as COPD or asthma (2,3,5,53).
The clinical and radiographic features of benign TBT cannot be often differentiated from those of malignant tumors. Many of these lesions have similar radiographic manifestations which are often non-specific and include atelectasis, pneumonia, bronchiectasis, and mediastinal shifts. Early recognition and diagnosis of these tumors may allow conservative treatment and excellent patient outcome (3,5).
Hamartomas
They are the most common type of benign endobronchial neoplasms (70% cases) with an incidence in general population between 0.025% and 0.32% (3). This tumor is most commonly located intrapulmonary and endobronchial location is very rare with an incidence of 1.4% in the largest review series (23,54). Tracheal hamartoma is extremely rare with less than 20 cases of adult found in literature (12,55). Tracheobronchial hamartoma are believed to be developmental in origin but more recent evidence points to a true neoplastic origin. Large series reported that hamartomas occur more frequently in older males with the peak in the sixth decade suggesting that inflammatory lung disease and smoking influence the incidence of hamartoma (3). These tumors are composed of a mixture of different tissues like cartilage, bone, fat and smooth muscle. Cartilaginous elements predominate and content of fat is higher in endobronchial than intraparenchymal lung hamartoma (13,23). Unlike pulmonary hamartomas, endobronchial hamartomas are often symptomatic. They usually manifested with symptoms of bronchial obstruction like cough, recurrent infection, wheezing, hemoptysis and dyspnea (2,53,56). Chest radiographic is non-specific and the two most common manifestations are consolidation and atelectasis caused by proximal airway obstruction. Endobronchial hamartoma appears on CT as a focal heterodense mass because of its various tissue components. CT findings such as internal fat and “popcorn” calcification together are typical of endobronchial hamartoma. However, nodules without these findings may be difficult for distinguishing from malignancies with CT alone. Postobstructive pneumonia or atelectasis can be present (Figure 8) (13,40,57). Hamartoma typically shows little or no radiotracer uptake on FDG PET. However, atypical pulmonary hamartoma may have increased FDG accumulation, thereby mimicking a malignancy. This false-positive finding could be explained by increased metabolic activity of the tumor (21). Bronchoscopic examination shows a polypoid or pedunculated sharply marginated mass with a smooth and yellowish surface without submucosal involvement (23,53).
Chondromas
Endobronchial chondromas are extremely rare benign tumor, which arises from the bronchial cartilage. As they have been historically misclassified as hamartomas, the true incidence of chondromas is still unknown. Endobronchial chondromas may be part of the Carney triad. Isolated endobronchial chondromas are less frequent than in Carney triade and can occur anywhere in the tracheobronchial tree with a higher prevalence in trachea (58). In Carney triad, chondroma affects young females whereas isolated chondromas affect males between third and fifth decade (53,58). Clinical manifestations of endobronchial chondroma depend on the extent of mechanical obstruction of bronchus. Symptoms are nonspecific, such as cough, sputum, fever, or dyspnea on exertion. Radiologic image is usually non-specific and the differential diagnosis includes hamartomas, tracheal amyloidosis, lung cancer and mucus plugs. The presence of bone and fat tissue on a chest CT suggests hamartoma. Well differentiated chondrosarcoma can be sometimes misdiagnosed as chondroma. The presence of tissue invasion, metastatic lesions are suggestive of chondrosarcoma or other metastasis (58-60).
Lipoma
Bronchial lipoma is extremely rare benign tumor with an incidence from 0.1% to 0.5% of all lung tumors and 1.4–13.0% of benign tumors of the lung (61). In a recent review of the English and Japanese literature on endobronchial lipomas, Fujino et al. reported 95 cases. An endobronchial location is twice as common as its parenchymal counterpart, and is often found in the left main-stem bronchus. Endobronchial lipomas consist of mature adipose tissue, some fibrous components lined with normal bronchial epithelium, sometimes developing squamous metaplasia (3,53,62). Largest series reported that endobronchial lipomas occur more frequently between the fifth and sixth decade of life onward, being more prevalent among males and obese persons (62). It has been reported that smoking and obesity are significant risk factors for endobronchial lipoma. The clinical symptoms of endobronchial lipoma are caused by airway obstruction and patients present with cough, hemoptysis, recurrent pneumonia, wheeze, or dyspnea. The duration of symptoms before diagnosis ranged from one month to few years (2,63). Radiographic finding is non-specific. In large series, most common chest X-ray and CT finding was atelectasis followed by consolidation (Figure 9). Other less frequent findings were tumor shadow, air trapping and pleural effusion. The size of the lesions reported in the literature varies from less than 1 cm to more than 7 cm (2,62,63). The CT findings of a homogeneous mass with fatty density and no tumor contrast enhancement are considered diagnostic (40,64). Magnetic resonance imaging enables accurate determination of the morphological characteristics of the mass with a hypersignal on the T1-weighted images and an intermediate signal on the T2-weighted images, compatible with normal fat. Endoscopically, the tumor appears as a soft gray, yellowish, mostly pedunculated mass that sometimes resists biopsy because of a firm capsule (12,23,53).
Mucous gland adenoma
Mucous gland adenoma is one of the rarest epithelial neoplasms which comprises of less than 0.5% of all lung tumors
Papilloma
Bronchial papillomas (BP) are rare TBT that account for 0.38% of all lung tumors and 7% to 8% of all benign lung tumors. Papillomas are normally classified into three separate categories: multiple papillomas, inflammatory polyps, and solitary papillomas. In adults, solitary BP are more common, prevalent in smokers, middle aged men (5,53). Multiple BP of the upper airways, often referred to juvenile laryngotracheal papillomatosis reflects neoplastic growth secondary to infection with human papillomavirus types 6 and 11 (68). They are more common in the pediatric population. Solitary BP of the bronchus is classified according to histologic features into three groups: squamous cell papilloma, glandular papillomas, and mixed (squamous cell and glandular) papillomas (68). They appear as a discrete polyploid nodule within the trachea, the lobar, or the segmental bronchi. The BP arises from the mucosal surface, and measure between 0.7 and 2.5 cm in diameter. They often cause narrowing of airway lumen which may lead to atelectasis, air trapping, post-obstructive infections, and bronchiectasis. The clinical history may last from a few months to two years of intermittent attacks of cough hemopthysis and dyspnea or history of repeated or unresolved pneumonia (68,69). The radiological manifestations depend on the size and location of the papilloma. Many of BP in trachea or main bronchi are not detected on the chest radiograph but CT may provide useful information. CT findings usually consist of a polypoid mass in the airway lumen and distal atelectasis and obstructive pneumonitis in the case of complete obstruction (Figure 10). Partial bronchial obstruction may result in reflex vasoconstriction leading to decreased perfusion and hyperlucency of the affected lung or lobe. Involvement of the distal airways and parenchyma can present with multiple, frequently cavitate nodules, measured up to several centimeters in diameter. Smoking, age above 40 years, and infections with human papillomavirus serotype 16 or 18 are risk factors for malignant transformation of benign squamous BP (70-72).
Primary endobronchial leiomyomas
Primary endobronchial leiomyomas (EL) are among the rarest of benign tumors of the respiratory tract accounting for approximately 0.66% of all benign lung neoplasms. These neoplasms can occur in lung parenchyma, trachea or bronchi. Endo-bronchial lesions constitute approximately 33% of all pulmonary leyomiomas (73). They are thought to derive from the smooth muscles of the bronchial tree. Origin from the areas of cicatricial fibrosis has also been proposed. Bronchial leiomyomas are benign tumors which predominantly occur in the third and fourth decade of life without gender predilection, although slight female preponderance has been reported in pulmonary leyomiomas (53,74). Patients with this condition have respiratory symptoms due to partial or complete airway obstruction which may stimulate asthma or be complicated with bronchiectasis and recurrent lung infection. Most common symptoms are cough, hemoptysis, dyspnea and malaise. Intermittent or constant dyspnea and wheezing are the most common symptoms of tracheal leiomyoma. The chest radiography may be normal in patients with small endobronchial tumor or shows solitary round mass or pneumonic infiltration, mediastinal shift, and collapse of lung to unilateral emphysema or hyperlucency according to the bronchial obstruction due to the tumor. On CT scans, tumor most commonly manifested as a homogeneously enhancing endo-luminal tumor with intraluminal growth. Sometimes, an iceberg appearance of the tumor (small intraluminal component and large extra-luminal component) can be found. Endobronchial nodule is often associated with post-obstructive pneumonia or atelectasis (53,73). Bronchosopy is greatly helpful in locating the exact site of the tumor and to obtain histopathological diagnosis. Tumors within the tracheobronchial tree appear as fleshy polypoid masses that protrude intraluminally and are attached to a wide base. A pre-operative histological diagnosis would certainly help in planning the site and resection of the tumor rather than radical resection of the lung.
Other endobronchial tumors
Other TBT are very rare and usually described as case reports or institutional experience. They include various tumors. Some of them originate from surface epithelium (large cell neuroendocrinal tumors), some originates from salivary glands (oncocytoma, myoepithelial carcinoma, pleomorphic adenoma, etc.) and some originates from mesenchyma (schwannoma, fibrosarcoma, chondrosarcoma, T-cell lymphoma). They are usually diagnosed incidentally on bronchoscopy or CT. They tend to have similar clinical presentation as cough, dispend, hemoptysis or signs of tracheobronchial obstruction (19).
Conclusions
A wide spectrum of tumors can occur in the tracheobronchial tree, including primary malignant tumors, secondary malignant tumors, and benign tumors. Except SCC and small cell lung cancer, tumors and tumorlike conditions of the central large airways are uncommon. Careful clinical evaluation, imaging and endoscopic examination are essential for the confirmation of endoluminal tracheobronchial lesions. Recent technical advances in immunohistochemistry and MDCT techniques (2D minimum-intensity-projection and 3D volume images) can very accurately define the location as well as intra- and extra-luminal extent of tracheal and endobronchial tumors. These techniques have become valuable tools for the clinician to assist with the diagnosis and planning of therapy for TBT.
Acknowledgements
Funding: This work was supported by the Ministry of Science and Technological Development of Serbia (Contract No. 175046).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Xu LT, Sun ZF, Li ZJ, et al. Clinical and pathologic characteristics in patients with tracheobronchial tumor: Report of 50 patients. Ann Thorac Surg 1987;43:276-8. [Crossref] [PubMed]
- Stevic R, Milenkovic B, Stojsic J, et al. Clinico-radiological characteristics of tracheobronchial tumors: Report of 65 cases. Ann Acad Med Singapore 2012;41:205-11. [PubMed]
- Wilson RW, Kirejczyk W. Pathological and radiological correlation of endobronchial neoplasms: Part I, benign tumors. Ann Diagn Pathol 1997;1:31-46. [Crossref] [PubMed]
- Wilson RW, Frazier AA. Pathological-radiological correlations: pathological and radiological correlation of endobronchial neoplasms: part II, malignant tumors. Ann Diagn Pathol 1998;2:31-4. [Crossref] [PubMed]
- Ko JM, Jung JI, Park SH, et al. Benign tumors of the tracheobronchial tree: CT-pathologic correlation. AJR Am J Roentgenol 2006;186:1304-13. [Crossref] [PubMed]
- Macchiarini P. Primary tracheal tumours. Lancet Oncol 2006;7:83-91. [Crossref] [PubMed]
- Takeda S, Hashimoto T, Kusu T, et al. Management and surgical resection for tracheobronchial tumors institutional experience with 12 patients. Interact Cardiovasc Thorac Surg 2007;6:484-9. [Crossref] [PubMed]
- Miller RD, Hyatt RE. Obstructing lesions of the larynx and trachea: clinical and physiologic characteristics. Mayo Clin Proc 1969;44:145-61. [PubMed]
- Miller RD, Hyatt RE. Evaluation of obstructing lesions of the trachea and larynx by flow-volume loops. Am Rev Respir Dis 1973;108:475-81. [PubMed]
- Hyatt RE, Black LF. The flow-volume curve: a current perspective. Am Rev Respir Dis 1973;107:191-9. [PubMed]
- Kadota N, Shinohara T, Machida H, et al. Asymptomatic tracheal MALT lymphoma discovered on spirometric findings presenting with elevated respiratory resistance. BMC Res Notes 2015;8:223. [Crossref] [PubMed]
- Wu CC, Shepard J-AO. Tracheal and airway neoplasms. Semin Roentgenol 2013;48:354-64. [Crossref] [PubMed]
- Ngo AV, Walker CM, Chung JH, et al. Tumors and tumorlike conditions of the large airways. AJR Am J Roentgenol 2013;201:301-13. [Crossref] [PubMed]
- Heidinger BH, Occhipinti M, Eisenberg RL, et al. Imaging of Large Airways Disorders. AJR Am J Roentgenol 2015;205:41-56. [Crossref] [PubMed]
- Laroia AT, Thompson BH, Laroia ST, et al. Modern imaging of the tracheo-bronchial tree. World J Radiol 2010;2:237-48. [Crossref] [PubMed]
- Jugpal TS, Garg A, Sethi GR, et al. Multi-detector computed tomography imaging of large airway pathology: A pictorial review. World J Radiol 2015;7:459-74. [PubMed]
- Allah MF, Hussein SR, El-Asmar AB, et al. Role of virtual bronchoscopy in the evaluation of bronchial lesions. J Comput Assist Tomogr 2012;36:94-9. [Crossref] [PubMed]
- Luo M, Duan C, Qiu J, et al. Diagnostic value of multidetector CT and its multiplanar reformation, volume rendering and virtual bronchoscopy. Postprocessing techniques for primary trachea and main bronchus tumors. PLoS ONE 2015;10:e0137329. [Crossref] [PubMed]
- Saoud M, Patil M, Dhillon SS, et al. Rare airway tumors: an update on current diagnostic and management strategies. J Thorac Dis 2016;8:1922-34. [Crossref] [PubMed]
- Gaissert HA, Mark EJ. Tracheobronchial gland tumors. Cancer Control 2006;13:286-94. [PubMed]
- Park CM, Goo JM, Lee HJ, et al. Tumors in the tracheobronchial tree: CT and FDG PET features. Radiographics 2009;29:55-71. [Crossref] [PubMed]
- Cho A, Hur J, Kang WJ, et al. Usefulness of FDG PET/CT in determining benign from malignant endobronchial obstruction. Eur Radiol 2011;21:1077-87. [Crossref] [PubMed]
- Pandey K, Vaidya PJ, Kate AH, et al. Bronchoscopic and surgical management of rare endobronchial tumors. J Cancer Res Ther 2016;12:1093-7. [Crossref] [PubMed]
- Wang LT, Wilkins EW, Bode HH. Bronchial carcinoid tumors in pediatric patients. Chest 1993;103:1426-8. [Crossref] [PubMed]
- Roby BB, Drehner D, Sidman JD. Pediatric tracheal and endobronchial tumors: an institutional experience. Arch Otolaryngol Head Neck Surg 2011;137:925-9. [Crossref] [PubMed]
- Compeau CG, Keshavjee S. Management of tracheal neoplasms. Oncologist 1996;1:347-53. [PubMed]
- Perelman MI, Koroleva N, Birjukov J, et al. Primary tracheal tumors. Semin Thorac Cardiovasc Surg 1996;8:400-2. [PubMed]
- Li W, Ellerbroek NA, Libshitz HI. Primary malignant tumors of the trachea: a radiologic and clinical study. Cancer 1990;66:894-9. [Crossref] [PubMed]
- Karam BM, Zahirifard S, Tahbaz MO, et al. Bronchial carcinoid tumors: Clinical and radiological findings in 21 patients. Iran J Radiol 2005;2:111-6.
- Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001;119:1647-51. [Crossref] [PubMed]
- Filosso PL, Rena O, Donati G, et al. Bronchial carcinoid tumors: Surgical management and long-term outcome. J Thorac Cardiovasc Surg 2002;123:303-9. [Crossref] [PubMed]
- Bueno Palomino A, Zurera Tendero L, Espejo Herrero JJ, et al. Multidetector computed tomography assessment of the degree of differentiation of bronchial carcinoid tumors. Radiologia 2013;55:323-30. [Crossref] [PubMed]
- Jeung MY, Gasser B, Gangi A, et al. Bronchial carcinoid tumors of the thorax: Spectrum of radiologic findings. Radiographics 2002;22:351-65. [Crossref] [PubMed]
- Hage R, de la Rivière AB, Seldenrijk CA, et al. Update in pulmonary carcinoid tumors: A review article. Ann Surg Oncol 2003;10:697-704. [Crossref] [PubMed]
- Luckraz H, Amer K, Thomas L, et al. Long-term outcome of bronchoscopically resected endobronchial typical carcinoid tumors. J Thorac Cardiovasc Surg 2006;132:113-5. [Crossref] [PubMed]
- Choplin RH, Kawamoto EH, Dyer RB, et al. Atypical carcinoid of the lung: Radiographic finding. AJR Am J Roentgenol 1986;146:665-8. [Crossref] [PubMed]
- Cardillo G, Sera F, Di Martino M. Bronchial carcinoid tumors: nodal status and long-term survival after resection. Ann Thorac Surg 2004;77:1781-5. [Crossref] [PubMed]
- Kim TS, Lee KS, Han J, et al. Sialadenoid tumors of the respiratory tract: radiologic-pathologic correlation. AJR Am J Roentgenol 2001;177:1145-50. [Crossref] [PubMed]
- Elnayal A, Moran CA, Fox PS, et al. Primary salivary gland-type lung cancer: imaging and clinical predictors of outcome. AJR Am J Roentgenol 2013;201:W57-63. [Crossref] [PubMed]
- Bhatia K, Ellis S. Unusual lung tumours: an illustrated review of CT features suggestive of this diagnosis. Cancer Imaging 2006;6:72-82. [Crossref] [PubMed]
- Maziak DE, Todd TR, Keshavjee SH, et al. Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg 1996;112:1522-31; discussion 1531-2. [Crossref] [PubMed]
- Albers E, Lawrie T, Harrell JH, et al. Tracheobronchial adenoid cystic carcinoma: a clinicopathologic study of 14 cases. Chest 2004;125:1160-5. [Crossref] [PubMed]
- Molina JR, Aubry MC, Lewis JE. Primary salivary gland-type lung cancer. Spectrum of clinical presentation, histopathologic and prognostic factor. Cancer 2007;110:2253-9. [Crossref] [PubMed]
- Huo Z, Meng Y, Wu H. Adenoid cystic ca of trachea. Int J Clin Exp Pathol 2014;7:7527-35. [PubMed]
- Cortés-Télles A, Mendoza-Posada D. Primary adenoid cystic carcinoma of the tracheobronchial tree: A decade-long experience at a health centre in Mexico. Lung India 2012;29:325-8. [Crossref] [PubMed]
- Vadasz P, Egervary M. Mucoepidermoid bronchial tumors: a review of 34 operated cases. Eur J Cardiothorac Surg 2000;17:566-9. [Crossref] [PubMed]
- Ha SY, Han J, Lee JJ, et al. Mucoepidermoid carcinoma of tracheobronchial tree: Clinicopathological study of 31 cases. Korean J Pathol 2011;45:175-81. [Crossref]
- Song Z, Liu Z, Zhang Y. Primary tracheobronchial mucoepidermoid carcinoma - a retrospective study of 32 patients. World J Surg Oncol 2013;11:62. [Crossref] [PubMed]
- Chopra A, Shim C, Sharma N, et al. Primary salivary type lung tumor: Mucoepidermoid carcinoma. Respir Med Case Rep 2013;9:18-20. [PubMed]
- Liu X, Adams AL. Mucoepidermoid carcinoma of the bronchus: A review. Arch Pathol Lab Med 2007;131:1400-4. [PubMed]
- Bai C, Huang Y, Li Q, et al. Clinical features of endotracheal/endobronchial metastases: analysis of 62 cases. Zhonghua Nei Ke Za Zhi 2007;46:806-9. [PubMed]
- Katsimbri PP, Bamias AT, Froudarakis ME, et al. Endobronchial metastases secondary to solid tumors: report of eight cases and review of the literature. Lung Cancer 2000;28:163-70. [Crossref] [PubMed]
- Agarwal A, Agrawal A, Alagusundarmoorthy SS, et al. Benign endobronchial neoplasms: A review. J Pulm Respir Med 2015;5:275.
- Gjevre JA, Myers JL, Prakash UB. Pulmonary hamartomas. Mayo Clin Proc 1996;71:14-20. [Crossref] [PubMed]
- Cetinkaya E, Gunluoglu G, Eyhan S, et al. A hamartoma located in the trachea. Ann Thorac Cardiovasc Surg 2011;17:504-6. [Crossref] [PubMed]
- Cosío BG, Villena V, Echave-Sustaeta J, et al. Endobronchial hamartoma. Chest 2002;122:202-5. [Crossref] [PubMed]
- Ahn JM, Im JG, Seo JW, et al. Endobronchial hamartoma: CT findings in three patients. AJR Am J Roentgenol 1994;163:49-50. [Crossref] [PubMed]
- Fernandez-Bussy S, Labarca G, Descalzi F, et al. Endobronchial chondromas. Respir Care 2014;59:e193-e196. [Crossref] [PubMed]
- Anrijs S, Weynand B, Pirson F, et al. Chondroma: an uncommon case of bronchial tumor. J Bronchology Interv Pulmonol 2009;16:270-3. [Crossref] [PubMed]
- Sen G, Tiwari M, Sraswat V. Pure endobronchial chondroma- A case report. Ind J Thorac Cardiovasc Surg 2008;24:215-7. [Crossref]
- Pollefliet C, Peters K, Janssens A, et al. Endobronchial lipomas: rare benign lung tumors, two case reports. J Thorac Oncol 2009;4:658-60. [Crossref] [PubMed]
- Muraoka M, Oka T, Akamine S, et al. Endobronchial lipoma: review of 64 cases reported in Japan. Chest 2003;123:293-6. [Crossref] [PubMed]
- Nataf P, Gharbi N, Bourcereau J, et al. Endobronchial lipomas. Apropos of 16 cases. Rev Pneumol Clin 1989;45:203-5. [PubMed]
- Yang YM, Pu C, Li Y, et al. Endobronchial lipoma: report of 2 cases and review of the Chinese literature. Zhonghua Jie He He Hu Xi Za Zhi 2012;35:176-9. [PubMed]
- England DM, Liselotte H. Truly benign "Bronchial Adenoma" Report of 10 cases of mucous gland adenoma with immunohistochemical and ultrastructural findings. Am J Surg Pathol 1995;19:887-99. [Crossref] [PubMed]
- Milenković B, Stojsić J, Mandarić D, et al. Mucous gland adenoma simulating bronchial asthma: case report and literature review. J Asthma 2007;44:789-93. [Crossref] [PubMed]
- Kwon JW, Goo JM, Seo JB, et al. Mucous gland adenoma of the bronchus: CT findings in two patients. J Comput Assist Tomogr 1999;23:758-60. [Crossref] [PubMed]
- Tryfon S, Dramba V, Zoglopiti F, et al. Solitary papillomas of the lower airways: Epidemiological, clinical, and therapeutic data during a 22-Year period and review of the literature. J Thorac Oncol 2012;7:643-8. [Crossref] [PubMed]
- McNamee CJ, Lien D, Puttagunta L, et al. Solitary squamous papillomas of the bronchus: a case report and literature review. J Thorac Cardiovasc Surg 2003;126:861-3. [Crossref] [PubMed]
- Paganin F, Prevot M, Noel JB, et al. A solitary bronchial papilloma with unusual endoscopic presentation: case study and literature review. BMC Pulm Med 2009;9:40. [Crossref] [PubMed]
- Barzó P, Molnár L, Minik K. Bronchial papillomas of various origins. Chest 1987;92:132-6. [Crossref] [PubMed]
- White SH, Ibrahim NB, Forrester-Wood CP, et al. Leiomyomas of the lower respiratory tract. Thorax 1985;40:306-11. [Crossref] [PubMed]
- Sharifi N, Fattahi Massoum SH, Karimi Shahr M, et al. Endobronchial leiomyoma; report of a case successfully treated by bronchoscopic resection. J Res Med Sci 2010;15:364-70. [PubMed]
- Kim YK, Kim H, Lee KS, et al. Airway leiomyoma: imaging findings and histopathologic comparisons in 13 patients. AJR Am J Roentgenol 2007;189:393-9. [Crossref] [PubMed]