Practical anesthetic considerations in patients undergoing tracheobronchial surgeries: a clinical review of current literature
Introduction and background
Anesthesia for tracheobronchial surgeries has always been challenging. Despite the anesthetic and surgical refinements there are still no randomized trials comparing various methods of anesthetic management among these surgeries. Anesthesia for tracheobronchial surgeries is especially challenging, as anesthesia provider and surgeon share a common area of intervention. Therefore, close and effective communication between the anesthesia and surgery partners is essential. Over the years, numerous attempts have been made to improve the practice and several sophisticated technical approaches have been defined for various complex surgeries and diagnostic procedures. Advance planning, meticulous check-lists for anesthesia equipment, anticipation of possible complications, and good communication as well as coordination between the teams involved are the key components to design a successful anesthetic plan.
The most common pathologies that require surgical intervention of the airways are post-intubation complications (accounting for 75% of cases) (1), neoplasms of the lungs or the airways and congenital airway anomalies.
The common patient characteristics presenting with tracheobronchial pathologies are either elderly with comorbid illness, compromised airways with tumors (post radiotherapy) or alternatively young patients with acute airway obstruction resulting from foreign body inhalation.
This review will provide an overview of safe anesthetic practices evolved over time for tracheobronchial surgeries and of the unique preparations required by anesthesia providers. Upper and lower tracheal surgeries require different approaches since algorithms pertaining to difficult airways do not apply once pathologies are distal to the vocal cords (2). The anticipated technical difficulties associated with these surgeries can be overcome by diligent pre-operative preparation.
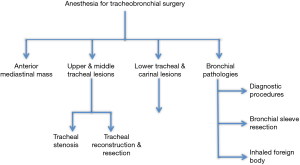
This review will describe the anesthetic management of the various surgical procedures of the tracheobronchial airway, as described in Figure 1.
Tracheal anatomy and pathophysiology
The origin of the trachea is defined as the inferior aspect of the cricoid cartilage at the approximate level of the sixth or seventh cervical vertebra. Various factors like obesity, flexibility of neck, body habitus and kyphosis may change the position and mobility of the trachea. The trachea maintains its structure with the rigid support of 18 to 24 C-shaped cartilaginous rings while the posterior wall of the trachea is constructed of a membranous band that lacks cartilaginous support. The distal margin of the carina bifurcates into the left and right main bronchi at the approximate level of the fifth thoracic vertebra. The trachea is classically divided into:
- Extra-thoracic trachea, above the suprasternal notch, which comprises approximately a 1/3 of its total length;
- Intra-thoracic trachea, extending from the suprasternal notch till the carina, accounting for the remaining 2/3 of its total length.
Freitag and colleagues have classified the causes of tracheal stenosis as either structural or dynamic (3).
- Structural (or fixed, intraluminal) stenosis occurs due to all types of exophytic intraluminal malignant or benign tumors and granulation tissue; extrinsic compression; narrowing due to airway distortion, kinking, bending, or buckling; and shrinking or scarring (e.g., post-intubation stenosis).
- Dynamic (or functional) stenosis is caused by a triangular-shaped or tent-shaped airway, in which cartilage is damaged, or alternatively an inward bulging of the posterior membranous wall (4).
The neoplastic growth usually follows a circumferential pattern, but resection along the same pattern is usually avoided during the surgery (5). The trachea is well supplied by the internal thoracic, intercostal and bronchial arteries through its lateral wall and might be vulnerable to ischemia during surgery.
The normal negative intrapleural pressure helps in providing stenting effect to the trachea and this effect is lost when the spontaneous respiration gets abolished with the induction of anesthesia.
Respiratory symptoms such as exertional dyspnea usually become evident once the tracheal lumen narrows to half its original diameter (6). Inspiratory stridor at rest indicates severe reduction in tracheal diameter.
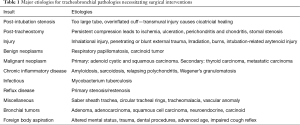
The major pathologies and their most common etiologies are detailed in Table 1. The most common indication for tracheal surgery is tracheal stenosis, with post-intubation stenosis being the most prevalent etiology. Prolonged compression of the tracheal mucosa by an overinflated cuff or an oversized endotracheal tube may cause obliteration of mucosal blood supply further leading to ischemia, ulceration, and the formation of granulation tissue and fibrosis causing contracture. As a clinical consequence, it is advisable to maintain cuff pressure below 30 mmHg (7).
Full table
General considerations in pre-operative assessment
Preoperative assessment of the airway does not always correlate with the ease of ventilation and, therefore, the practice of managing airways varies among experts. The location of the stenosis could be divided into three regions: upper third, middle third, and lower third of the trachea.
Detailed medical history and physical examination with special consideration to airways and pulmonary systems, recent respiratory tract infections, exercise tolerance, patients preferred positions without short of breath, amount of pulmonary secretions and previous endotracheal intubations as well as demand of oxygen during the day at several activities should be noted. Neck mobility examination prior to surgery is essential as it is reported to facilitate both intra-operative exposure and post-operative healing (8). History of chronic steroid exposure and radiation therapy is important as it can interfere with wound healing and may cause wound dehiscence (9).
Clinical signs and symptoms remain the most important set of information for evaluating a patient for tracheal surgeries. Nevertheless, the gold standard for evaluation of stenosis is formal bronchoscopy (10). The severity of airflow resistance correlates to the 4th power of the tracheal radius, assuming laminar flow (resistance α radius4, Poiseuille’s Equation). The pattern of dyspnea (or stridor) can be helpful in localizing the site of stenosis. Stridor and cyanosis are late signs and usually indicate near-total obstruction. The positions that alleviate or worsen airway obstruction are of crucial importance for the anesthesia provider and may become lifesaving during unexpected anesthetic events. Radiologic imaging is useful adjunct in assessing airways. Stenosis quantification done by CT scans and bronchoscopy have almost comparable results (11-13).
Pulmonary function tests might be helpful in predicting the likelihood of post-operative ventilatory dependence in the setting of pulmonary dysfunction and in monitoring the progress of obstruction after treatment (14-16). The clinical relevance of flow volume loops is questionable. Although not considered as a good predictor during usual preoperative evaluation, they might aid in identification of the inspiratory and expiratory irregularities or cause (fixed or dynamic obstruction) of tracheal stenosis (17).
Fixed/intraluminal stenosis causes decrease in the flow and plateau parts of both the inspiratory and expiratory limbs of the flow-volume loop, whereas extrathoracic obstruction usually only influences the flow in the inspiratory limb.
General intraoperative considerations
The overall anesthetic goal is to maintain a patent airway, allow maximal surgical exposure, and have the patient fully awake and cooperative at the end of surgery.
The therapeutic options available for tracheal pathologies are tracheal reconstruction, laser or electro-cautery excision, tracheal dilation and stenting. The method of ventilation depends on the level of lesions and degree of airway obstruction, as detailed in Table 2. Spontaneous ventilation is better tolerated in intrathoracic lesions (18). This allows the patient to maintain airway patency using muscle tone and respiratory efforts.

Full table
The apneic oxygenation as described by Patel under the name of THRIVE (19) may well play an important role in the future, where the inner lumen of the trachea remains open enough. This oxygenation technique needs proper validation in the context of tracheobronchial surgery.
Anesthesia management of anterior mediastinal masses (AMMs)
AMMs can be benign (thymoma, cyst, thyroid mass, cystic hygroma) or malignant (thyroid/thymic carcinoma, lymphoma).
These masses often encroach on and exert pressure effects on the middle mediastinum causing severe respiratory and cardiovascular compromise leading to death (20,21). Although mortality pertaining to surgery and anesthesia is considered low, AMMs can cause compressive symptoms that might be life threatening, further exacerbated by general anesthesia. Mortality directly related to AMMs is reported as higher in the pediatric age group, possibly due to the compressible nature of the cartilaginous airways (22,23). New imaging modalities have almost eradicated the use of general anesthesia for diagnostic purposes.
Pre-anesthetic assessment involves focused history with special attention to local symptoms related to the mass and its pressure effects on intrathoracic organs. Superior vena cava obstruction requires special attention. Severe symptomatic superior vena cava syndrome (usually manifested as facial and upper extremities edema) may require cardiopulmonary bypass or extracorporeal membrane oxygenation cannulation prior to induction and good venous access in the lower extremities (24).
CT imaging may provide vital information, including the site and severity of the mass effect as well as its relationship to the airway. General anesthesia causes reduction in functional residual capacity, relaxation of smooth muscles and loss of spontaneous diaphragmatic movements, all of which precipitate decrease in transpleural pressure gradient and, thereby, promote airway and vascular collapse under the AMM’s pressure (25). Spontaneous ventilation exerts some protective stenting effect on the trachea, explaining why imaging done during full consciousness cannot predict airway or even vascular effects seen once the patient is exposed to general anesthesia, and more so to muscle relaxants (25).
Echocardiogram is mandatory for patients with suspected cardiovascular collapse. Flow volume loops used to be a part of routine pre-anesthesia evaluation as a predictor of “variable intrathoracic airway obstruction” (25) but were shown to have a limited correlation with airway obstruction (26). Nevertheless, post-operative complications can be predicted to some extent by two preoperative factors: tracheal compression >50%, and peak expiratory flow <40% of predicted value (27). Preexisting endocrine abnormalities, prior exposure to chemo-radiation and associated paraneoplastic syndromes must also be considered in the setup of AMMs.
Patients with large AMMs that cross the midline require sternotomy, and, therefore, placement of high thoracic epidural prior to induction should be considered in order to alleviate post-operative pain. Induction in a stepwise manner coupled with strict hemodynamic monitoring to detect early signs of cardiovascular collapse and intermittent blood gas analyses are common practices. Optimal positioning is crucial during induction. Dyspnea in the supine position warrants an upright posture during induction.
This strategy is helpful in stabilizing the patient and may become lifesaving while seeking an alternative plan. Patients with high risk of cardiovascular collapse may be maintained on spontaneous ventilation and avoiding muscle relaxants until definitive airway is established. This can be achieved by the administration of inhalational agents or IV medications such as propofol, remifentanil, dexmedetomidine (28) and others. Judicious use of short acting muscle relaxants and assisted ventilation with the aim of providing positive pressure ventilation might alternatively be considered. Awake fiberoptic intubation may be necessary if imaging shows non-compressible tracheal lesions.
The following strategies should be considered in the event of catastrophic airway or vascular collapse during anesthesia:
- Posture change—optimal posture determined by the preoperative evaluation;
- Ventilation via a rigid bronchoscope;
- Lightening the plane of anesthesia and awakening the patient;
- Emergent sternotomy and elevation of the mass.
Confirmation of dynamic airway compression using fiberoptic bronchoscopy may also be considered if the clinical situation allows.
To overcome severe cardiovascular compromise, the use of cardiopulmonary bypass might be indicated (29). It should be emphasized, though, that cardiopulmonary bypass is hardly ever feasible if adequate preparations were not done ahead of time. Therefore cardiopulmonary bypass should not merely be considered as “on standby”, but rather cannulation should be performed, usually under local anesthesia. Tubing should be primed before induction of anesthesia, to be prepared for anticipated hemodynamic or respiratory collapse.
Emergency management of tracheal stenosis
No definitive management is defined for the treatment of stenosis. The most effective immediate solution for stenosis includes endoscopic intervention by rigid bronchoscope and simultaneous administration of steroids, oxygen, racemic adrenaline and diuretics to decrease edema surrounding the lesion and relieve obstruction.
Mechanical dilation by balloon dilators
This approach transiently improves oxygenation by relieving the obstruction.
Tracheal dilation and stenting
The tracheal stenosis primarily follows circumferential pattern of growth, therefore emergent airways techniques like tracheostomy, cricothyroidectomies, trans-tracheal ventilation would not be beneficial.
The primary aim of stenting is to provide symptomatic relief in the conditions where the lesion is obstructing and surgery is practically impossible to perform. Mediastinal masses causing central airway obstruction may also benefit with this intervention as stents serve as bridge to palliative treatment. Providing anesthesia to this population is more challenging as it requires increased attention to local and systemic effects of malignancies like superior vena cava syndrome, endocrine abnormalities, fibrosis from previous radiotherapies, cardiovascular compromise from chemotherapy (30). The important surgical steps involved in tracheal stenting are identification of lesion, guide wire placement, threading of balloon over the guide wire and dilation of stenosis.
Lesions present below the vocal cords rarely presents with difficult intubation because of tracheal pathologies per se. However, distal lesion acts as dynamic obstruction and interfere with expiratory flow leading to “air trapping” of distal segments. Allowing adequate expiratory time by keeping a threshold on inspiratory pressures below 30 cmH2O is a good strategy (31).
Tracheal stent itself is a stimulus to cause airway obstruction. Coughing should be avoided as it may cause total obstruction. At presentation, patients should receive supplemental humidified oxygen and maintain upright position, this simple maneuvers helps to avoid coughing.
Surgical approaches involve the use of endoscopic procedures. Maintenance of airways is crucial in both rigid and flexible bronchoscope procedures. The use of both rigid and flexible bronchoscope has been described in the literature and both approaches.
Rigid bronchoscope insertion is preferable and quicker to achieve as it has an advantage of secured airways with good surgical exposure (31). Total intravenous anesthesia involving the use of analgesics, hypnotics and anti-secretory agents is preferred as volatile anesthetic delivery cannot be guaranteed. There is always a risk of aspiration with the use of rigid bronchoscope, so patients with full stomach, hiatal hernias, morbid obesity should be weighed with risks and benefits. Mouth guard should be used to prevent injury. Rigid bronchoscope is inserted after complete neuromuscular blockade and instillation of intermittent jet ventilation via side port. The four basic ventilation methods are (32):
- Spontaneous ventilation with inhalational anesthetic along with topical anesthesia or nerve block;
- Apneic oxygenation method (allows surgical intervention for 3 minutes or longer) with or without oxygen insufflation;
- Intermittent positive pressure ventilation (IPPV) with bronchoscopic ventilation;
- Jet ventilation during total intravenous anesthesia.
The complications associated with rigid bronchoscope are: airway perforation, hypoxemia, hemorrhage, airway edema. In patients where there is risk of potential airway loss after the procedure it is always prudent to intubate a patient with smaller ETT after rigid bronchoscopy.
The use of local anesthesia and inhalational techniques precipitate coughing and should be avoided (31). The most crucial steps are placement of dilator and stents over the stenosis. Newer expandable stents allows more room for the manipulation and they open up when they are positioned well in the stenosed segment.
A flexible bronchoscope can be threaded through the endotracheal tube or the laryngeal mask airway (LMA) where a rigid bronchoscope cannot be negotiated. The ET can be placed under sedation and tip of tube should be placed just above the stenosis. This approach involves insertion of ventilatory conduits such as catheters through stenosed segment. Stent placement may be difficult if the obstruction is distal and may complicate the surgical field by tissue laceration, bleeding, and perforation.
During surgeries requiring the use of Nd:YAG (neodymium-doped yttrium aluminium garnet) laser, the inspired oxygen should be kept as low as possible (usually oxygen should not exceed 30%) to prevent airways fires.
Tracheal reconstruction and resection
Advance preparation is the key for successful management. Various size endotracheal tubes (cuffed and un-cuffed), microlaryngeal tubes, emergency cricothyroidotomies kits should be ready in the cart. Standard ASA monitors are used and further requirements depends on case to case basis and coexisting comorbidities. Wide bore IV cannula in bilateral upper limb is needed as upper extremities are usually tucked in and are inaccessible for the anesthesia provider during ongoing surgery. Ability to perform bronchoscopy and jet ventilation instillation should be handy in “cannot intubate” conditions (33). Blood pressure monitoring is usually done by left radial artery catheterization and pulse oximetry on the other side. Since the innominate artery is close to the trachea, compression of the trachea and subsequently the artery may cause conflicting readings. Identification of brachiocephalic compression is essential as confusion about actual hypotensive state and compression of brachiocephalic vein can be distinguished.
Initially the patient is placed in extended neck position to enable enough space for surgical manipulation and later on changed to neutral position to exert minimal tension on the anastomotic segment of trachea.
Maintaining adequate oxygenation and ventilations is necessary during all surgical partial sections. The optimal ventilatory approach is defined by the location of lesion in the trachea.
Prolonged and adequate pre-oxygenation during obtained spontaneous breathing and carefully titrated induction of anesthesia is obligatory indicated. Assistance to generate adequate tidal volumes is sometimes indicated during pre-oxygenation and should be performed as carefully and as convenient as possible for the patient.
Rigid bronchoscopic evaluation just after induction is sometimes necessary to look for exact location of the lesion to be resected, evidence of tracheomalacia and vocal cord mobility (34).
Jet ventilation via bronchoscope allows surgeon to reduce tissue load by serial dilation and restore the diameter of trachea which is followed by tracheal intubation. The potential side complication is barotrauma and hypercarbia (32). Total intravenous anesthesia is an acceptable approach with intermediate acting muscle relaxant. Judicious use of small titrated doses of opioids is advocated keeping in mind the goal of extubation after surgery and in the same time provide adequate analgesia.
The distal tracheal intubation technique is done when upper and middle trachea surgery is planned. The ETT is pulled back and the care must be taken not to puncture the cuff when the incision is made by the surgeon. The distal segment is intubated with another ETT inserted by surgeon in sterile technique and confirmed by EtCO2. Care must be taken to reduce the use of inhalational anesthesia as large amount of leak is expected during “open airway portion” of procedure to avoid anesthetic pollution. A laryngeal mask can also be used before the trachea is opened. Gas exchange during this time is performed in principle by one of these techniques: (I) jet ventilation; (II) IPPV and distal segment tracheal intubation; (III) manual ventilation; (IV) extracorporeal membrane oxygenation or cardiopulmonary bypass, which is performed in only high-specialized clinics. Anesthesia is primarily maintained by intravenous method during this phase.
Once the anastomosis is done and is ready to close, the upper trachea is again intubated with a separate ET in retrograde fashion. The cuff is positioned under fiberoptic visualization in such a way that it is distal to the sutures or site of lesion. Before complete closure, the surgical site should carefully examined to detect any bleeding or leakage.
A rapid test can be to check any leak in the tracheal anastomosis by deflating the cuff applying 30 cm of water pressure to the breathing circuit and spilling saline over the suture site. Look for bubbles as an evidence of air leak. Guardian sutures are placed to prevent excessive hyper extension of neck. Use of anti-emetic at this point would be helpful in reducing nausea. After emergence the patient neck should be in fully flexed position and positioned in semi sitting posture and ETT is removed under fibroptic visualization when the patient is fully awake. Supplemental oxygen should be given.
Emergence from anesthesia is the most critical step. Smooth extubation and prevention from coughing and even extensive patients movement is strictly indicated.
Coughing and pressure effects of ETT on the reconstructed segment have serious implications like anastomotic failure. Placing the patient in head up and neck flexed position with the help of guardian suture (suture between skin of mandible and chest) helps to improve ventilation. Assessment of recurrent laryngeal nerve is essential after surgery.
Lower tracheal and carinal resections may be more conveniently approached by right thoracotomy. Arterial blood pressure monitoring is essential for all thoracotomies as it involves release of the right hilar ligament which can cause compression of the cardiopulmonary system and decreased perfusion pressure. The anesthetic management is similar to upper tracheal lesions except the fact that emergency surgical access to the airway is not possible. A variety of airway devices can be used in an attempt to pass the low obstruction, e.g., long single lumen tubes, bronchial blockers and jet ventilation (35). Long lasting and/or technically extensively challenging tracheal reconstructions can be done on extracorporeal membrane oxygenation as it ensures stable hemodynamics and oxygen supply (36), but again is limited to highly specialized clinics..
Endobronchial tubes placed under fiberoptic guidance are preferred to double lumen tubes as the latter are too bulky to facilitate surgical procedure. Endobronchial jet catheters may be placed via an ETT and if necessary, differential lung ventilation can be achieved using two catheters.
Single lung ventilation is usually needed in order to provide optimal surgical exposure. All patients undergoing single lung ventilation usually have a variable level of shunt. Independent lung ventilation can be utilized in cases of continuous desaturation, but might interfere with the surgical procedure. Other options include:
- Deflating the cuff of endobronchial tube and blocking the nose and mouth while delivering large tidal volumes;
- Placing an additional endotracheal tube high up in trachea;
- A laryngeal mask can be placed after the endotracheal tube is in place and provide a seal to deliver sufficient tidal volumes;
- Jet catheter can also be placed in the deflated lung.
Postoperative pain management is essential as the thoracotomy itself is considered a large and painful intervention (37). Thoracic epidural catheters are commonly used for post-operative pain control. Judicious use of systemic opioid agents is also possible, as long as respiratory depression is avoided. Prolonged surgeries requiring lower tracheal or carinal resection usually require post op ventilation for up to 24 hours.
Patients with previous history of lower tracheal or carinal surgeries who are undergoing other surgeries should usually be carefully intubated under fiberoptic guidance, to avoid tissue damage or malpositioning due to altered anatomy.
Anesthesia for bronchial surgeries and procedures
The advancement of diagnostic and therapeutic bronchial procedures in recent years with more sophisticated navigation capabilities, such as endobronchial ultrasound guided fine-needle-aspiration and electro-magnetic navigation bronchoscopy, has necessitated the development of modern anesthesia management tools as well (33). These procedures are usually performed in remote locations by their mere definition, with their inherent anesthetic challenges of scarce rescue and emergency resources. Another serious concern is the usually impaired physical status of the patients. These patients may suffer from severe co-morbidities including for example bronchial tumors or chronic airway infections with limited pulmonary function.
Another subset of patients that are indicated for urgent bronchoscopy are young children and vulnerable adults who present to emergency room with suspected foreign body aspiration. The presenting symptoms can be stridor, dyspnea, dysphagia, hemoptysis or signs of hypoxemia. Imaging may reveal collapse or consolidation distal to the foreign body or emphysematous distention.
Premedication in the form of anxiolytics should be carefully considered in very anxious patients, and might be especially important in the pediatric population. The need for monitoring after anxiolytics should be assessed and planned accordingly. Supplemental oxygen and anti-sialagogues are routinely administered.
The different anesthetic alternatives for bronchoscopic interventions are: (I) general anesthesia; (II) conscious sedation; (III) monitored anesthesia care.
Preferred techniques for most of the prolonged bronchial procedures are general anesthesia with total intravenous anesthesia. Intermediate acting muscle relaxants are indicated as the surgery requires introduction of a bronchoscope through the vocal cords. Muscle relaxants will also prevent excessive coughing and laryngospasm.
Anesthesia for bronchial sleeve resection
This procedure involves removal of a circular sleeve in the main bronchus leaving lung parenchyma intact, and is considered for patients with insufficient cardiopulmonary reserve indicated for pneumonectomies. As previously mentioned, pulmonary function tests might predict post-operative ventilatory dependence (14). Double lumen tubes or bronchial blockers are the common devices used for lung isolation. The “Open lung” approach warrants the need for thoracic epidural for post-operative pain management. If a video-assisted thoracoscopic surgery is planned, paravertebral block can be considered (38).
Anesthesia for Inhaled foreign body
This is the leading cause of accidental death in the population younger than 4 years of age (39,40). Almost 80% of foreign bodies are made of organic material. Classic symptoms include dyspnea, stridor, aphonia, cyanosis, cough or total obstruction. Supraglottic foreign bodies usually present with inspiratory wheeze while infraglottic bodies will produce expiratory wheeze. History of a witnessed choking event is suggestive of aspiration.
Decreased breath sounds, rhonchi, wheeze, non-resolving pneumonia are typically late findings of aspiration. A foreign body can impede airflow by four mechanisms: (I) inspiratory one-way valve—air may be inhaled but not exhaled; (II) expiratory one-way (“ball”) valve—air may be exhaled but not inhaled; (III) bypass valve—partial obstruction of inhalation and exhalation; (IV) stop valve—total blockage.
Depending upon hemodynamic and respiratory stability of patient, chest radiograph can be obtained to locate the position of the foreign body assuming it is made of radio-opaque material. Tracheal objects tend to align in the sagittal plane (40). Secondary evidence for the presence of a foreign body comprises of atelectasis, air trapping, emphysema or mediastinal shift (41). However, chest radiographs can be normal in 17% of cases or it may not show any evidence during the first 24 hours (40). Lateral radiographs are helpful in identifying objects behind the trachea which reflect as soft tissue swelling and loss of cervical lordosis. CT scan and virtual bronchoscope are more sensitive compared to chest radiographs (42,43). Traditional rigid bronchoscopy can be considered for diagnostic, but also for therapeutic purposes. Two prospective studies compared the use of rigid compared to flexible bronchoscopy in the treatment of foreign body aspiration. Both studies concluded, that patients with clinical signs of asphyxia in combination with radiological findings like evidence of a foreign body, emphysema, and unilateral decreased breath sounds should undergo rigid bronchoscopy (44,45). In contrast, another retrospective study reported a success rate of 91% of removing foreign bodies by flexible bronchoscopy during a observational period of 8 years and treating 1,027 patients (46). Overall, rigid bronchoscope provides better visualization, control and ventilation whereas the flexible bronchoscope is better suited for foreign bodies in more distal areas due to its flexibility.
However, the choice of rigid or flexible bronchoscopy should be more dependent on personal experience and preference.
Anesthetic considerations
A quick pre-operative assessment followed by imaging might assist in locating the FB. If distal to the carina, the risk of total obstruction is smaller. Organic matter like vegetables or nuts absorb water and swells whereas sharp objects can pierce the airway. Document the time since the last meal. Orogastric tube can be used for aspiration of gastric contents. Time management is crucial, and obvious signs of complete obstruction will indicate an urgent bronchoscopic intervention under anesthesia.
Maintenance of spontaneous ventilation is theoretically important as conversion to positive pressure ventilation can dislodge foreign body causing complete obstruction. Nevertheless, a small prospective trial of 36 patients showed controlled ventilation is more effective than spontaneous breathing (47). Prevention of bucking and coughing is essential. Traditionally, a volatile anesthetic agent (sevoflurane) is preferred over intravenous agents for induction (48), but recently with the development of newer short acting intravenous anesthetics are also gaining popularity, although their use might be associated with increased incidence of laryngospasm and breath holding (49-51). Once adequate depth of anesthesia is reached, a rigid bronchoscope is inserted through the glottic opening. The anesthesia circuit may be attached to the bronchoscope’s side arm to allow ventilation (52).
After extraction of the foreign body, the decision to intubate will be guided by the clinical impression of the mucosal membranes—if edema or obstruction is evident, preventive intubation can be performed.
Flexible bronchoscopy can also be utilized in selected cases, and be performed under sedation and topical anesthesia. In uncooperative patients, though, it may require general anesthesia with supra or infraglottic airway control.
Summary
Surgical interventions in the airway can be extremely challenging for the anesthesia provider, with various considerations that change according to the type and duration of the procedure, anatomical location, patient’s comorbidities and the urgency. Meticulous planning and preparing for unexpected events, along with detailed knowledge of the surgical plan are probably the most important factors in the successful administration of anesthesia for these challenging cases. Close cooperation and clear communication between anesthesia and surgery providers remains the most important key to success.
Acknowledgements
The authors want to thank Dr. Abdelmalak Basem for his helpful support during the initial phase of writing this clinical review.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Grillo HC, Donahue DM. Post intubation tracheal stenosis. Semin Thorac Cardiovasc Surg 1996;8:370-80. [PubMed]
- Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013;118:251-70. [Crossref] [PubMed]
- Freitag L, Ernst A, Unger M, et al. A proposed classification system of central airway stenosis. Eur Respir J 2007;30:7-12. [Crossref] [PubMed]
- Faust RA, Remley KB, Rimell FL. Real-time, cine magnetic resonance imaging for evaluation of the pediatric airway. Laryngoscope 2001;111:2187-90. [Crossref] [PubMed]
- Sandberg W. Anesthesia and airway management for tracheal resection and reconstruction. Int Anesthesiol Clin 2000;38:55-75. [Crossref] [PubMed]
- Al-Bazzaz F, Grillo H, Kazemi H. Response to exercise in upper airway obstruction. Am Rev Respir Dis 1975;111:631-40. [PubMed]
- Touat L, Fournier C, Ramon P, et al. Intubation-related tracheal ischemic lesions: incidence, risk factors, and outcome. Intensive Care Med 2013;39:575-82. [Crossref] [PubMed]
- Pinsonneault C, Fortier J, Donati F. Tracheal resection and reconstruction. Can J Anaesth 1999;46:439-55. [Crossref] [PubMed]
- Mathisen DJ. Complications of tracheal surgery. Chest Surg Clin N Am 1996;6:853-64. [PubMed]
- Finkelstein SE, Schrump DS, Nguyen DM, et al. Comparative evaluation of super high-resolution CT scan and virtual bronchoscopy for the detection of tracheobronchial malignancies. Chest 2003;124:1834-40. [Crossref] [PubMed]
- Koletsis EN, Kalogeropoulou C, Prodromaki E, et al. Tumoral and non-tumoral trachea stenoses: evaluation with three-dimensional CT and virtual bronchoscopy. J Cardiothorac Surg 2007;2:18. [Crossref] [PubMed]
- Perotin JM, Jeanfaivre T, Thibout Y, et al. Endoscopic management of idiopathic tracheal stenosis. Ann Thorac Surg 2011;92:297-301. [Crossref] [PubMed]
- Morshed K, Trojanowska A, Szymański M, et al. Evaluation of tracheal stenosis: comparison between computed tomography virtual tracheobronchoscopy with multiplanar reformatting, flexible tracheofiberoscopy and intra-operative findings. Eur Arch Otorhinolaryngol 2011;268:591-7. [Crossref] [PubMed]
- Bernstein WK, Deshpande S. Preoperative evaluation for thoracic surgery. Semin Cardiothorac Vasc Anesth 2008;12:109-21. [Crossref] [PubMed]
- Melendez JA, Barrera R. Predictive respiratory complication quotient predicts pulmonary complications in thoracic surgical patients. Ann Thorac Surg 1998;66:220-4. [Crossref] [PubMed]
- Ranu H, Wilde M, Madden B. Pulmonary function tests. Ulster Med J 2011;80:84-90. [PubMed]
- Ranu H, Madden BP. Endobronchial stenting in the management of large airway pathology. Postgrad Med J 2009;85:682-7. [Crossref] [PubMed]
- Hobai IA, Chhangani SV, Alfille PH. Anesthesia for tracheal resection and reconstruction. Anesthesiol Clin 2012;30:709-30. [Crossref] [PubMed]
- Patel A, Nouraei SA. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia 2015;70:323-9. [Crossref] [PubMed]
- Ng A, Bennett J, Bromley P, et al. Anaesthetic outcome and predictive risk factors in children with mediastinal tumours. Pediatr Blood Cancer 2007;48:160-4. [Crossref] [PubMed]
- Bittar D. Respiratory obstruction associated with induction of general anesthesia in a patient with mediastinal Hodgkin's disease. Anesth Analg 1975;54:399-403. [PubMed]
- Victory RA, Casey W, Doherty P, et al. Cardiac and respiratory complications of mediastinal lymphomas. Anaesth Intensive Care 1993;21:366-9. [PubMed]
- Takeda S, Miyoshi S, Akashi A, et al. Clinical spectrum of primary mediastinal tumors: a comparison of adult and pediatric populations at a single Japanese institution. J Surg Oncol 2003;83:24-30. [Crossref] [PubMed]
- Kokotsakis J, Chaudhry UA, Tassopoulos D, et al. Surgical management of superior vena cava syndrome following pacemaker lead infection: a case report and review of the literature. J Cardiothorac Surg 2014;9:107. [Crossref] [PubMed]
- Neuman GG, Weingarten AE, Abramowitz RM, et al. The anesthetic management of the patient with an anterior mediastinal mass. Anesthesiology 1984;60:144-7. [Crossref] [PubMed]
- Torchio R, Gulotta C, Perboni A, et al. Orthopnea and tidal expiratory flow limitation in patients with euthyroid goiter. Chest 2003;124:133-40. [Crossref] [PubMed]
- Béchard P, Létourneau L, Lacasse Y, et al. Perioperative cardiorespiratory complications in adults with mediastinal mass: incidence and risk factors. Anesthesiology 2004;100:826-34; discussion 5A.
- Abdelmalak B, Makary L, Hoban J, et al. Dexmedetomidine as sole sedative for awake intubation in management of the critical airway. J Clin Anesth 2007;19:370-3. [Crossref] [PubMed]
- Tempe DK, Arya R, Dubey S, et al. Mediastinal mass resection: Femorofemoral cardiopulmonary bypass before induction of anesthesia in the management of airway obstruction. J Cardiothorac Vasc Anesth 2001;15:233-6. [Crossref] [PubMed]
- Nethercott D, Strang T, Krysiak P. Airway stents: anaesthetic implications. Contin Educ Anaesth Crit Care Pain 2010;10:53-8. [Crossref]
- Conacher ID. Anaesthesia and tracheobronchial stenting for central airway obstruction in adults. Br J Anaesth 2003;90:367-74. [Crossref] [PubMed]
- Pathak V, Welsby I, Mahmood K, et al. Ventilation and anesthetic approaches for rigid bronchoscopy. Ann Am Thorac Soc 2014;11:628-34. [Crossref] [PubMed]
- Chadha M, Kulshrestha M, Biyani A. Anaesthesia for bronchoscopy. Indian J Anaesth 2015;59:565-73. [Crossref] [PubMed]
- Ramsay MA, Saha D, Hebeler RF. Tracheal resection in the morbidly obese patient: the role of dexmedetomidine. J Clin Anesth 2006;18:452-4. [Crossref] [PubMed]
- Arthur ME, Odo N, Parker W, et al. CASE 9--2014: Supracarinal tracheal tear after atraumatic endotracheal intubation: anesthetic considerations for surgical repair. J Cardiothorac Vasc Anesth 2014;28:1137-45. [Crossref] [PubMed]
- Rosskopfova P, Perentes JY, Ris HB, et al. Extracorporeal support for pulmonary resection: current indications and results. World J Surg Oncol 2016;14:25. [Crossref] [PubMed]
- Hughes R, Gao F. Pain control for thoracotomy. Contin Educ Anaesth Crit Care Pain 2005;5:56-60. [Crossref]
- Kaya FN, Turker G, Mogol EB, et al. Thoracic paravertebral block for video-assisted thoracoscopic surgery: single injection versus multiple injections. J Cardiothorac Vasc Anesth 2012;26:90-4. [Crossref] [PubMed]
- Weissberg D, Schwartz I. Foreign bodies in the tracheobronchial tree. Chest 1987;91:730-3. [Crossref] [PubMed]
- Fidkowski CW, Zheng H, Firth PG. The anesthetic considerations of tracheobronchial foreign bodies in children: a literature review of 12,979 cases. Anesth Analg 2010;111:1016-25. [PubMed]
- Pinto A, Scaglione M, Pinto F, et al. Tracheobronchial aspiration of foreign bodies: current indications for emergency plain chest radiography. Radiol Med 2006;111:497-506. [Crossref] [PubMed]
- Hong SJ, Goo HW, Roh JL. Utility of spiral and cine CT scans in pediatric patients suspected of aspirating radiolucent foreign bodies. Otolaryngol Head Neck Surg 2008;138:576-80. [Crossref] [PubMed]
- Koşucu P, Ahmetoğlu A, Koramaz I, et al. Low-dose MDCT and virtual bronchoscopy in pediatric patients with foreign body aspiration. AJR Am J Roentgenol 2004;183:1771-7. [Crossref] [PubMed]
- Martinot A, Closset M, Marquette CH, et al. Indications for flexible versus rigid bronchoscopy in children with suspected foreign-body aspiration. Am J Respir Crit Care Med 1997;155:1676-9. [Crossref] [PubMed]
- Righini CA, Morel N, Karkas A, et al. What is the diagnostic value of flexible bronchoscopy in the initial investigation of children with suspected foreign body aspiration? Int J Pediatr Otorhinolaryngol 2007;71:1383-90. [Crossref] [PubMed]
- Tang LF, Xu YC, Wang YS, et al. Airway foreign body removal by flexible bronchoscopy: experience with 1027 children during 2000-2008. World J Pediatr 2009;5:191-5. [Crossref] [PubMed]
- Soodan A, Pawar D, Subramanium R. Anesthesia for removal of inhaled foreign bodies in children. Paediatr Anaesth 2004;14:947-52. [Crossref] [PubMed]
- Kain ZN, O'Connor TZ, Berde CB. Management of tracheobronchial and esophageal foreign bodies in children: a survey study. J Clin Anesth 1994;6:28-32. [Crossref] [PubMed]
- Sersar SI, Rizk WH, Bilal M, et al. Inhaled foreign bodies: presentation, management and value of history and plain chest radiography in delayed presentation. Otolaryngol Head Neck Surg 2006;134:92-9. [Crossref] [PubMed]
- Chen LH, Zhang X, Li SQ, et al. The risk factors for hypoxemia in children younger than 5 years old undergoing rigid bronchoscopy for foreign body removal. Anesth Analg 2009;109:1079-84. [Crossref] [PubMed]
- Litman RS, Ponnuri J, Trogan I. Anesthesia for tracheal or bronchial foreign body removal in children: an analysis of ninety-four cases. Anesth Analg 2000;91:1389-91. TOC. [Crossref] [PubMed]
- Sullivan MT, Neff WB. A modified Sanders ventilating system for rigid-wall bronchoscopy. Anesthesiology 1979;50:473-4. [Crossref] [PubMed]