The application of autologous pulmonary artery in surgical correction of complicated aortic arch anomaly
Introduction
Aortic arch anomalies with intracardiac defects are common congenital heart diseases, including coarctation of aorta, aortic arch hypoplasia and interruption of aortic arch with ventricular septal defect. End-to-end anastomosis is routinely applied to reconstruct descending aortic arch in the clinical settings. However, in the cases of severe aortic arch hypoplasia, longer aortic coarctation segment or distance of interruption of aortic arch, end-to end anastomosis may cause a series of postoperative complications, such as anastomotic stenosis, bronchial obstruction, and bleeding caused by the large tension of anastomosis. Therefore in such cases, homograft vessel or autologous pericardial patch is applied to augment descending aortic arch. Nevertheless, autologous pericardial patch is not an optimal material to repair aortic arch anomalies because of the lack of elasticity of normal vessel. In addition, homograft vessel with the drawbacks of calcification of homograft, potential antigen-antibody reaction, degeneration long-term after transplantation, often results in severe aortic regurgitation or stenosis and needs reoperation (1,2). Besides, homograft vessel is always difficult to obtain. Consequently, some researchers (3,4) suggested that autologous main pulmonary artery could be applied in the reconstruction of descending aortic arch. In this study, we reported primary experience of surgical correction of complicated aortic arch anomaly with autologous main pulmonary artery in our center.
Methods
From July 2010 to March 2016, autologous main pulmonary artery was used to reconstruct descending aortic arch in twenty-one patients in our center. The five patients were diagnosed as interrupted aortic arch with ventricular septal defect and patent ductus arteriosus (age: 20 days old, 5 months old, 2, 5 and 16 years old), of these three are male patients and two are females with a median age of 2 years. The median weight was 9.8 kg (range, 3.2–40 kg). While sixteen patients were diagnosed as coarctation of aorta (coarctation segment was equal to or more than 2 cm). The eleven patients are males and the other five are females. The median age is 7.8 months (range, 1 month –5 years) and median weight is 8.4 kg (range, 3.5–17.5 kg).
Preoperative echocardiography and CT scan were performed in all patients. All patients underwent one-stage surgical repair via median sternotomy alone. Cardiopulmonary bypass was established with arterial cannulation of both ascending aorta and descending aorta in patients with interrupted aortic arch and single cannulation of ascending aorta in other cases. Aortic arch anomaly was reconstructed under deep hypothermia, including deep hypothermic circulatory arrest (DHCA) in 15 patients, and deep hypothermic continuous low flow bypass with selective cerebral perfusion in 6 patients. Intracardiac anomaly was repaired when cooling to deep hypothermia (18 °C).
For 5 patients with interrupted aortic arch, because of older age, poorer elasticity of artery tissue and longer distance of interrupted aortic arch, it is difficult and risky to perform end-to-end or end-to-side anastomosis under careful consideration. Therefore, anterior wall of dilated main pulmonary artery was used to repair aortic arch anomalies. Then anterior wall of main pulmonary artery was excised to form a conduit whose diameter (range from 7 to 20 mm) varied according to the patient’s body surface area. Finally both ends of conduit were anastomosed to descending aorta and aortic arch, respectively (Figure 1).
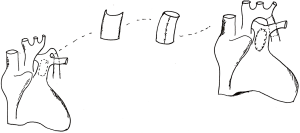
For 16 patients with coarctation of aorta with ventricular septal defect, because of longer coarctation segment (more than 20 mm), it is impossible to perform tension-free repair when resecting all the coarctation segment and arterial ductus ligament. Therefore, it was usual to establish partial connection of descending aortic arch firstly and augment aortic arch with tailored pulmonary artery patch in oval shape. Finally, the defect of main pulmonary artery was repaired with autologous pericardial patch (Figure 2) (5). After reconstructing descending aorta, arterial cannula was reinserted or adjusted, and systemic circulation was restored and aortic cross clamp was open after de-airing. The remaining procedure was the same as other general cardiopulmonary bypass operations.

Results
Patients
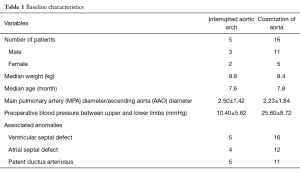
The characteristics of 21 patients are shown in Table 1. As shown here, the five patients were diagnosed as interrupted aortic arch with ventricular septal defect and patent ductus arteriosus. Additionally, four of them possess atrial septal defect as well. The other sixteen patients were diagnosed as coarctation of aorta mostly complicating ventricular septal defect—atrial septal defect and patent ductus arteriosus. The ratio of pulmonary arterial diameter and aortic diameter in the patients with interrupted aortic arch was 2.5±1.42, and 2.23±1.84 in the patients with coarctation of aorta. The obvious preoperative blood pressure between upper and lower limbs could be observed in the two groups of patients, 10.4±5.62 mmHg in the patients with interrupted aortic arch and 25.6±8.72 mmHg in coarctation of aorta.
Full table
Outcomes
In this study, one patient died of multiple organ failure who had suffered anasarca and oliguria before surgery, and the other patients survived without any neurologic complications. One of patients underwent reoperation for anastomotic bleeding, and there were four patients delayed sternal closure. The average cardiopulmonary bypass time was 116 minutes. The average aortic cross clamp time was 62 minutes. The average DHCA time was 29.5 minutes. The average span of chest tubes placement was 44.8 hours. The average of mechanical ventilation time was 18.6 hours. The average of ICU monitoring time was 4.6 days. The average length of hospital stay was 13.6 days.
Follow-up
The duration of follow-up was from second to forty-fourth month and median was the twenty-fourth month. During the follow-up period, the differences of blood pressure between upper and lower limbs or bronchial obstruction was not significant. Additionally, the ratio of main pulmonary arterial diameter and ascending aorta diameter were 1.23±1.37 and 1.05±1.14 in patients with interrupted aortic arch and patients with coarctation of aorta respectively. For two patients with interrupted aortic arch during the follow-up, cardiac CT scan and cardioangiography showed that the descending aortic arch had good physiological curve and the diameter of aortic arch increased by almost 25% compared with that in the surgery. The echocardiography for all patients in 3, 6, 12, 24 months after surgery showed that blood flow in the descending aortic arch was fluent and there was no obvious blood pressure gradient. New York Heart Association (NYHA) classification of all patients was class I.
Discussion
Aortic arch anomalies with intracardiac defects are common congenital heart diseases, including coarctation of aorta, aortic arch hypoplasia or interruption of aortic arch with ventricular septal defect. During last two decades, the treatment of congenital heart diseases made great progress. With the improvement of technique of the myocardial protection and DHCA, the patients with this kind of congenital heart diseases had favorable clinical outcome (6-8). In the clinical settings, end-to-end anastomosis is used to reconstruct descending aortic arch. However, in the cases of severe aortic arch hypoplasia, longer aortic coarctation segment or distance of interruption of aortic arch, end-to-end anastomosis may lead to anastomotic stenosis, bronchial obstruction and bleeding due to the large tension of anastomosis. The incidence of aortic restenosis is from 5% to 25% (9,10). In order to solve these problems, surgeons applied autologous pericardial patch, homograft or xenograft vessel or resected left subclavian artery to augment descending aortic arch. Furthermore, unlike infants, for older children, it is difficult to perform end-to-end anastomosis even with extensive dissection and repair completely aortic arch hypoplasia because of the decrease of elasticity of vessel.
Autologous pericardial patch is not an optimal material to repair aortic arch anomalies because of the lack of elasticity of normal vessel. Besides, uneven tensions of vessel wall after surgery may result in the formation of aneurysm (11,12). Therefore, cardiac surgeons in western countries preferred homograft with the elasticity of normal vessel to reconstruct descending aortic arch. However, homograft vessel has the calcification of homograft, potential antigen-antibody reaction, lacking durability. In addition, homograft vessel is rare and difficult to obtain.
Some researchers (1,2) suggested that autologous substitute may be optimal to reconstruct descending aortic arch. Importantly, as artery tissue, autologous main pulmonary artery has excellent elasticity and even tension; meanwhile, autologous main pulmonary artery can’t lead to antigen-antibody reaction; furthermore, it’s easy to harvest autologous substitute without any managements; additionally, autologous main pulmonary artery has growth potentiality; moreover, the harvest of autologous substitute won’t damage pulmonary valve and annulus, which can retain the growth capacity of pulmonary artery; and finally, the application of autologous main pulmonary artery can’t result in large anastomotic tension, which decreases the incidence of bronchial obstruction and anastomotic restenosis.
In this study, infants with aortic arch anomalies and intracardiac defects had severe main pulmonary artery pressure, and the diameter of main pulmonary artery was at least 2.5 fold larger than that of aorta. Usually, the older infants were, the larger the ratio of diameter of main pulmonary artery and aorta was, and what was even worse, the ratio of diameter of main pulmonary artery and aorta was as high as four. Dilated main pulmonary artery can lead to left main bronchial obstruction, therefore, it’s necessary to perform pulmonary arterioplasty. And this pathophysiological procedure made it possible for us to apply main pulmonary artery tissue. In the operation, we used anterior wall of main pulmonary artery to reconstruct descending aortic arch while retaining lateral and posterior wall of main pulmonary artery and pulmonary valve and annulus, and the defect of anterior wall of main pulmonary artery was repaired with pericardial patch. Like Ross operation, in this procedure, autologous substitute was used while retaining the growth capacity of main pulmonary artery. Transverse incisions were made 4 mm above sinotubular junction and in front of arterial duct respectively, and longitudinal incisions were made near the left and right pulmonary orifices. Aortic arch was augmented with tailored main pulmonary artery patch in oval shape. And for those with longer distance of interrupted aortic arch, we excised anterior wall of main pulmonary artery to form a conduit whose diameter (range from 7 to 20 mm) varied according to the area of patient’s body surface, and then both ends of conduit were anastomosed to descending aorta and aortic arch respectively. This procedure was feasible and could achieve “Cut and Use It” without any managements.
One year post the transplantation of autologous main pulmonary artery, the ratio of main pulmonary arterial diameter to ascending aorta diameter was close to 1:1 in the two groups of patients, which indicated that pulmonary artery pressure gradually returned to normal. Two patients (5 months and 2 years old) with interrupted aortic arch (the diameter of pulmonary artery was 10 and 20 mm, respectively), cardiac CT scan and cardioangiography showed that the shape of aortic arch was normal and diameter of aortic arch increased by about 3 and 5 mm, respectively, which indicated that autologous main pulmonary artery had potential growth capacity. For other cases where autologous main pulmonary artery was used to reconstruct descending aortic arch, during the follow-up, we found no differences of blood pressures between upper and lower limbs, no left main bronchial obstruction, no anastomotic tension, no distortion of main pulmonary artery and no pulmonary regurgitation, and bleeding was well controlled.
Conclusions
Taken together, autologous pulmonary artery can be applied in surgical correction of complicated aortic arch anomaly with ventricular septal defect. This procedure is feasible and has favorable early and mid-term outcomes, and autologous pulmonary artery can retain growth capacity during follow-up. However, long-term follow-up is necessary to draw an accurate conclusion of this technique.
Acknowledgements
Funding: This work was supported by the grant from National Science and Technology Support Program of China (2011BAI11B00).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Guangdong General Hospital [No. 2011135H(R3)].
References
- Danesi TH, Minniti G, Cresce GD, et al. Redo After Failure of Aortic Homografts With a Rapid Deployment Valve. Ann Thorac Surg 2016;102:e281-2. [Crossref] [PubMed]
- Chang Q, Jing H, Sun M, et al. Exploring the role of short-course cyclosporin a therapy in preventing homograft valve calcification after transplantation. Cell Immunol 2014;287:36-45. [Crossref] [PubMed]
- Bergoënd E, Bouissou A, Paoli F, et al. A new technique for interrupted aortic arch repair: the Neville tube. Ann Thorac Surg 2010;90:1375-6. [Crossref] [PubMed]
- Roussin R, Belli E, Lacour-Gayet F, et al. Aortic arch reconstruction with pulmonary autograft patch aortoplasty. J Thorac Cardiovasc Surg 2002;123:443-8; discussion 449-50. [Crossref] [PubMed]
- Lee H, Yang JH, Jun TG, et al. Augmentation of the Lesser Curvature With an Autologous Vascular Patch in Complex Aortic Coarctation and Interruption. Ann Thorac Surg 2016;101:2309-14. [Crossref] [PubMed]
- Kanter KR, Mahle WT, Kogon BE, et al. What is the optimal management of infants with coarctation and ventricular septal defect? Ann Thorac Surg 2007;84:612-8; discussion 618. [Crossref] [PubMed]
- Morales DL, Scully PT, Braud BE, et al. Interrupted aortic arch repair: aortic arch advancement without a patch minimizes arch reinterventions. Ann Thorac Surg 2006;82:1577-83; discussion 1583-4. [Crossref] [PubMed]
- Alsoufi B, Cai S, Coles JG, et al. Outcomes of different surgical strategies in the treatment of neonates with aortic coarctation and associated ventricular septal defects. Ann Thorac Surg 2007;84:1331-6; discussion 1336-7. [Crossref] [PubMed]
- Walhout RJ, Lekkerkerker JC, Oron GH, et al. Comparison of polytetrafluoroethylene patch aortoplasty and end-to-end anastomosis for coarctation of the aorta. J Thorac Cardiovasc Surg 2003;126:521-8. [Crossref] [PubMed]
- Wright GE, Nowak CA, Goldberg CS, et al. Extended resection and end-to-end anastomosis for aortic coarctation in infants: results of a tailored surgical approach. Ann Thorac Surg 2005;80:1453-9. [Crossref] [PubMed]
- Marcheix B, Lamarche Y, Perrault P, et al. Endovascular management of pseudo-aneurysms after previous surgical repair of congenital aortic coarctation. Eur J Cardiothorac Surg 2007;31:1004-7. [Crossref] [PubMed]
- Brown ML, Burkhart HM, Connolly HM, et al. Late outcomes of reintervention on the descending aorta after repair of aortic coarctation. Circulation 2010;122:S81-4. [Crossref] [PubMed]


