Risk factors and outcome of primary graft dysfunction after lung transplantation in Korea
Introduction
Primary graft dysfunction (PGD) is a form of acute lung injury (ALI) that occurs within the first few days after allograft reperfusion in lung transplant recipients. The incidence of PGD is known to be 10–30% and it is the major cause of mortality within the first post-transplant year (1,2). PGD necessitates prolonged mechanical ventilation and a longer stay in the intensive care unit (ICU), and leads to poor functional outcomes and an increased risk of bronchiolitis obliterans syndrome (1,2). PGD grade 3 is associated with worse clinical outcomes than other PGD grades regardless of time point (3,4). Therefore, the identification of risk factors for PGD, especially PGD grade 3, is very important for improving the outcomes of lung transplantation patients.
Several studies have attempted to identify clinical risk factors for PGD after lung transplantation, but have given conflicting results, possibly as a result of different sample sizes, inconsistencies in PGD definition, and a failure to consider multiple clinical factors (4-6). Since 2005, when the International Society for Heart and Lung Transplantation (ISHLT) published guidelines for PGD, its definition has become more consistent, although differences of clinical factors associated with PGD between centers can still remain (1,4,5,7).
Studies of PGD after lung transplantation are particularly limited in Asia, despite the growing use of this procedure, and we have therefore sought to identify clinical risk factors for PGD and to study the outcomes of PGD in order to improve the care of lung transplantation patients in Korea.
Methods
Study design and population
We performed a retrospective study using data collected between January 2010 and March 2014 in one tertiary care hospital in South Korea. Sixty-one patients aged ≥18 years who underwent single or bilateral lung transplantation were performed lung transplantation during study period and all the patients were enrolled.
Lung transplantation
- Method of lung harvesting, preservation solution, anterograde and/or retrograde perfusion. All organs were recovered en bloc and organ was obtained from mechanically assisted brain-dead donors. For preservation solution, low-potassium dextran solution (Perfadex®; Duraent Biologicals, Hyderabad, India) was used. Anterograde and retrograde flushing were used.
- Technique of implantation: clamshell incision in fourth intercostal space was used in double lung transplantation. Anterolateral thoracotomy was also used. When double lung transplantation was performed, right side organ was usually implanted first.
- Criteria for extracorporeal life support (CPB vs. ECMO): patients with preoperative ECMO underwent surgery with ECMO support, but if the patient could not tolerate with ECMO support, CPB was instituted. When the patient was hemodynamically unstable or the patient cannot tolerate to pulmonary artery clamping or single-lung ventilation, CPB was instituted.
Definition of PGD grading
We graded PGD according to the ISHLT criteria, based on the partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio and the presence of diffuse parenchymal infiltrates in the allograft on a chest radiograph (1,3,8). And we diagnosed PGD after excluding other diagnosis. As for infection such as pneumonia, clinical situation and parameters such as CRP, fever and sputum characteristics were used. And to rule out vascular anatomic complication, clinical situation was considered and if needed, imaging modalities such as ultrasonography was used. The primary outcome was the development of grade 3 PGD (PaO2/FiO2 ratio <200) at 48 or 72 hours after transplantation, and the clinical outcome after grade 3 PGD.
Variables
The evaluated factors of recipients were age, gender, body mass index (BMI) (kg/m2), preoperative diagnosis, the use of extracorporeal membrane oxygenation (ECMO) before lung transplantation, echocardiographic parameters before lung transplantation, smoking history, transplantation type, the use of cardiopulmonary bypass (CPB) during surgery, and the intraoperative ischemic time. Ischemic time was defined as the ischemic period between the initiation of the aorta cross clamp to the start of cold perfusion. The clinical data of donors were also evaluated, including age, gender, BMI, smoking history, heart function as assessed by echocardiography, oxygenation, and duration of ventilator use. Participants were classified into five BMI groups according to the World Health Organization’s criteria for Asia-Pacific populations (9): BMI <18.5, 18.5≤ BMI <23.0, 23.0≤ BMI <25.0, 25.0≤ BMI <30.0, and 30.0≤ BMI.
Clinical outcome
We evaluated the clinical outcome after PGD which was evaluated by re-operation, postoperative ventilator days, postoperative ICU stay, acute kidney injury, need for renal replacement therapy (RRT) after transplantation, tracheostomy, and postoperative hospital stay. The definition of re-operation was the operation which was performed after transplantation due to any cause. The cause of re-operation included the bleeding, empyema, bronchopleural fistula, wound dehiscence and pericardial effusion.
Statistical analysis
Data are described as numbers (percentages) or medians [interquartile ranges (IQRs)]. We compared variables using the Mann-Whitney or Chi-square test. Potential risk factors for grade 3 PGD previously identified in the literature and shown by univariate analysis in our study or with hypothetical clinical or biologic plausibility were selected for analysis (1-8,10-18). A logistic regression model was used to identify the independent variables associated with grade 3 PGD. A two-tailed P value <0.05 was considered statistically significant. The PASW Statistics 18 software package (IBM, Armonk, New York, USA) was used for all analyses.
Ethics
The research protocol was approved by the Institutional Review Board (IRB) of Severance Hospital (IRB No. 4-2013-0770).
Results
A total of 61 patients who received lung transplantation were enrolled. A total of 16 subjects (26.2%) met criteria for grade 3 PGD at the first 48 or 72 hours after transplantation.
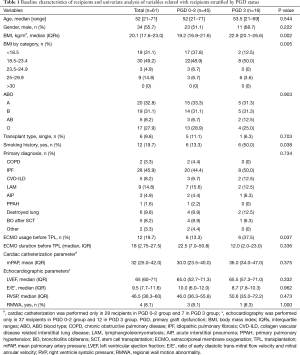
Baseline characteristics of recipients and univariate analysis of variables related to recipients stratified by PGD status are presented in Table 1. Median age (grade 0–2 PGD vs. grade 3 PGD: 52 years, range 21–71 years vs. 53.5 years, 21–69 years; P=0.544) and the proportion of male patients (51.1% vs. 68.7%; P=0.222) were not significantly different between the grade 0–2 and grade 3 PGD groups. Idiopathic pulmonary fibrosis was the most common diagnosis before transplantation in both groups (44.4% vs. 50.0%, P=0.734). A higher BMI (median: 19.2, IQR 16.9–21.6 vs. median: 22.9, IQR 20.1–26.6, P=0.002), any history of smoking (13.3% vs. 50.0%, P=0.038), and ECMO use before transplantation (13.3% vs. 37.5%, P=0.037) were significantly more common in the grade 3 than in the grade 0–2 PGD group.
Full table
The univariate analyses of variables related to the donor and operation are summarized in Tables 2 and 3. There were no donor-related variables associated with grade 3 PGD. Age, gender, BMI, smoking history, PaO2/FiO2 ratio before transplantation, the duration of ventilator use, the type of transplantation, and intraoperative cardiopulmonary support were not associated with grade 3 PGD. Only the total intraoperative ischemic time was significantly longer in the grade 3 PGD group than in the grade 0–2 PGD group (median: 350.0 min, IQR 319.0–396.0 min vs. 307.0 min, IQR: 283.3–363.3 min, P=0.012).

Full table

Full table
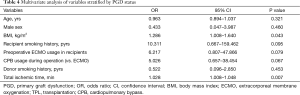
Multivariate analysis revealed that a high BMI in recipients [odds ratio (OR), 1.286; 95% confidence interval (CI), 1.008–1.640; P=0.043] and a longer intraoperative ischemic time (OR, 1.028; 95% CI, 1.008–1.048; P=0.007) were independent risk factors for grade 3 PGD (Table 4).
Full table
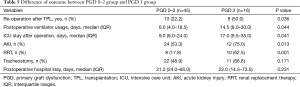
The outcomes of grade 0–2 and grade 3 PGD patients are summarized in Table 5. Grade 3 PGD was significantly associated with a higher re-operation rate (grade 0–2 PGD vs. grade 3 PGD: 22.2% vs. 50.0%; P=0.036), prolonged ventilation (median: 6.0 days, IQR 4.0–18.5 days vs. 14.5 days, IQR: 8.0–30.0 days; P=0.044), a longer ICU stay (median: 9.0 days, IQR: 6.0–24.0 days vs. 17.0 days, 9.5–35.0 days; P=0.041), and a higher rate of RRT (17.8% vs. 62.5%; P=0.001) after transplantation.
Full table
Discussion
In this study, we identified risk factors for grade 3 PGD at 48 or 72 hours after lung transplantation, and assessed the outcome of these patients. A higher BMI in recipients and a longer total intraoperative ischemic time were associated with grade 3 PGD after lung transplantation, and the outcome of grade 3 PGD patients was poorer than that of patients with grade 0–2 PGD with respect to the rate of re-operation, the duration of ventilator treatment, the length of ICU stay, and the rate of RRT.
PGD is a rather common complication, occurring in 10–30% of lung transplant recipients (1,8). Diamond et al. found that the incidence of grade 3 PGD varied (2–27%) across multiple centers in the US, although a consistent definition of PGD was used (5). They concluded that the distribution of risk factors, the lack of a standardized treatment approach, and differences in patient characteristics may have contributed to this variation. In our study, the incidence of grade 3 PGD was 26.2%. Although the total number of lung transplantations in Korea is relatively small, the incidence of grade 3 PGD was equivalent to that reported in some previous studies, suggesting that similar factors were associated with grade 3 PGD.
Some studies reported that donor smoking history, alcohol consumption, being overweight, a single lung transplantation, use of CPB during the operation, preoperative medical history of sarcoidosis or pulmonary artery hypertension, and higher preoperative mean pulmonary artery pressure were risk factors for PGD after lung transplantation (1,2,10-13,19). Other studies found that female gender and the use of transfusion were risk factors for the development of PGD (5,7).
In this study, a higher BMI was significantly associated with grade 3 PGD, which concurs with the findings of a previous report in which overweight and obese patients were enrolled (12). However, because the proportion of overweight and obese patients in our study was low, caution is required in the interpretation of its results. Underweight patients have many problems in transplantation, nevertheless, their risk of grade 3 PGD tend to be low compared to normal body weight patients in our study. Possible mechanisms through which a higher BMI increases the risk of grade 3 PGD are leptin-mediated lung inflammation and the production of pro-inflammatory cytokines from adipose tissues and macrophages (12,20,21).
A longer intraoperative ischemic time was also found to be a risk factor for grade 3 PGD in our study. Previous studies have had conflicting findings in this respect. Thabut and colleagues found that cold ischemic time was associated with poor outcomes among patients with PGD (14), whilst other groups reported that there was no association between ischemic time and PGD (5,8,15,16). There could be a threshold ischemic time for the development of significant ischemic reperfusion injury, and thus the development of PGD might vary between centers that have different mean intraoperative ischemic times. However, the exact relationship between ischemic time and PGD is not fully understood. Future research into these underlying mechanisms may lead to improved preventive strategies.
Although not statistically significant in multivariate analysis, factors such as smoking history in recipients (13.3% vs. 50.0%; P=0.038) and the use of ECMO before transplantation (13.3% vs. 37.5%; P=0.037) were associated with grade 3 PGD in univariate analysis. Smoking and the use of ECMO can induce systemic inflammation and ECMO may be related to a poor general condition of the recipient. Thus, systemic inflammation or the recipient’s general condition may be associated with PGD after transplantation.
The outcome of the grade 3 PGD group was poorer than that of the grade 0–2 PGD group, in agreement with previous studies (5,17,18). Although a longer stay in ICU and longer ventilator use in the grade 3 PGD group were consistent with other studies, a higher rate of re-operation, acute kidney injury, and RRT in grade 3 PGD patients has not been reported before. This may be the result of severe hypoxia in grade 3 PGD, or possibly the poorer condition of recipients as the rate of ECMO usage before transplantation was higher in the grade 3 PGD group.
This study had a number of strengths and limitations. The strengths includes the fact that this is the first study of PGD after lung transplantation in Asia, and our findings reveal that the incidence, risk factors, and outcome of PGD were relatively similar to those reported by previous international reports. Limitations include the use of only one center, the potential for bias, and the exclusion of factors such as final mortality. Second, we were confused in excluding edema and infection from PGD in some patients. However, several methods were used to differentiate PGD from other conditions. To exclude pneumonia, symptoms such as fever or increased sputum, culture, C-reactive protein, and response to antibiotics were monitored. And echocardiography was routinely used to exclude cardiogenic pulmonary edema and pulmonary venous outflow obstruction. Third, factors such as intraoperative transfusion and elevated mean pulmonary artery pressure in donors, which are known risk factors for PGD, were not fully analyzed due to limited information. Finally, this study was relatively small and retrospective in nature. Further prospective studies are needed that include greater patient numbers.
In conclusion, we have identified risk factors associated with the development of grade 3 PGD and assessed the outcome of grade 3 PGD after lung transplantation. Patients who developed grade 3 PGD had higher re-operation rate, longer ventilator apply, longer intensive care unit stay, higher rate of RRT, with higher BMI and total intraoperative ischemic time being the significant risk factor. Our findings could be used to develop predictive models to help physicians to modify identifiable risk factors prior to the development of PGD.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board (IRB) of Severance Hospital (No. 4-2013-0770).
References
- Christie JD, Carby M, Bag R, et al. Report of the ISHLT working group on primary lung graft dysfunction part II: definition—a consensus statement of the international society for heart and lung transplantation. J Heart Lung Transplant 2005;24:1454-9. [Crossref] [PubMed]
- Lee JC, Christie JD, Keshavjee S. Primary graft dysfunction: definition, risk factors, short- and long-term outcomes. Semin Respir Crit Care Med 2010;31:161-71. [Crossref] [PubMed]
- Christie JD, Bellamy S, Ware LB, et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant 2010;29:1231-9. [Crossref] [PubMed]
- Suárez López VJ, Miñambres E, Robles Arista JC, et al. Primary graft dysfunction after lung transplantation. Med Intensiva 2012;36:506-12. [PubMed]
- Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527-34. [Crossref] [PubMed]
- Nosotti M, Palleschi A, Rosso L, et al. Clinical risk factors for primary graft dysfunction in a low-volume lung transplantation center. Transplant Proc 2014;46:2329-33. [Crossref] [PubMed]
- Liu Y, Liu Y, Su L, et al. Recipient-related clinical risk factors for primary graft dysfunction after lung transplantation: a systematic review and meta-analysis. PLoS One 2014;9:e92773. [Crossref] [PubMed]
- Christie JD, Kotloff RM, Pochettino A, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest 2003;124:1232-41. [Crossref] [PubMed]
- WHO/IASO/IOTF. The Asia-Pacific perspective: redefining obesity and its treatment. Health Communications Australia: Melbourne; 2000.
- Fang A, Studer S, Kawut SM, et al. Elevated pulmonary artery pressure is a risk factor for primary graft dysfunction following lung transplantation for idiopathic pulmonary fibrosis. Chest 2011;139:782-7. [Crossref] [PubMed]
- Shah RJ, Diamond JM, Cantu E, et al. Objective estimates improve risk stratification for primary graft dysfunction after lung transplantation. Am J Transplant 2015;15:2188-96. [Crossref] [PubMed]
- Lederer DJ, Kawut SM, Wickersham N, et al. Obesity and primary graft dysfunction after lung transplantation: the lung transplant outcomes group obesity study. Am J Respir Crit Care Med 2011;184:1055-61. [Crossref] [PubMed]
- Lowery EM, Kuhlmann EA, Mahoney EL, et al. Heavy alcohol use in lung donors increases the risk for primary graft dysfunction. Alcohol Clin Exp Res 2014;38:2853-61. [Crossref] [PubMed]
- Thabut G, Vinatier I, Brugière O, et al. Influence of preservation solution on early graft failure in clinical lung transplantation. Am J Respir Crit Care Med 2001;164:1204-8. [Crossref] [PubMed]
- Gammie JS, Stukus DR, Pham SM, et al. Effect of ischemic time on survival in clinical lung transplantation. Ann Thorac Surg 1999;68:2015-9; discussion 2019-20.
- Fiser SM, Kron IL, Long SM, et al. Influence of graft ischemia time on outcomes following lung transplantation. J Heart Lung Transplant 2001;20:206-207. [Crossref] [PubMed]
- Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant 2007;26:1004-11. [Crossref] [PubMed]
- Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2007;175:507-13. [Crossref] [PubMed]
- Hayes D Jr, Galantowicz M, Yates AR, et al. Venovenous ECMO as a bridge to lung transplant and a protective strategy for subsequent primary graft dysfunction. J Artif Organs 2013;16:382-5. [Crossref] [PubMed]
- Faggioni R, Fantuzzi G, Fuller J, et al. IL-1 beta mediates leptin induction during inflammation. Am J Physiol 1998;274:R204-8. [PubMed]
- Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995;269:540-3. [Crossref] [PubMed]


