|
Molecular mechanisms of EMT
The major characteristic of EMT is the conversion from epithelial cells to motile, invasive and migratory mesenchymal
cells. In the process, epithelial cells lose cell-cell adhesion and
cell polarity, decrease the expression of epithelial cells marker
such as E-cadherin, increase the expression of mesenchymal
cell markers such as Vimentin, fibronectin, N-cadherin, alphasmooth
muscle actin (α-SMA), as well as increase the activity
of matrix metalloproteinases (MMPs) like MMP-2, MMP-3
and MMP-9, associated with invasive phenotype ( 7). Thus,
cancer cells acquire the capacity to migrate and invade the
surrounding stroma and subsequently spread through the blood
and lymphatic vessels to distant site. The conversion of epithelial cells to mesenchymal cells is coordinately regulated by a number
of signaling pathways, transcriptional factors, eventually resulting
in the loss of epithelial markers and acquistion of mesenchymal
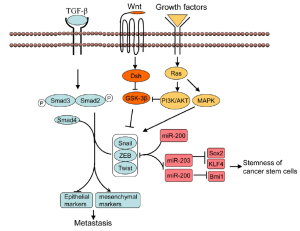
features ( Fig 1).
EMT and signaling pathways
EMT can be induced by various signal factors, including TGF- β,
growth factors that act through receptor tyrosine kinases such as
fibroblast growth factor, hepatic growth factor, and Wnt, Notch
and hedgehog proteins. Among these signaling factors, TGF-β
is the most extensively studied inducer of EMT. TGF-β binding to its receptors leads to the activation of Smad2 and Smad3
through direct C-terminal phosphorylation by TGF-β receptor
I, phosphorylated Smad2 and Smad3 then form trimers with
Smad4, Smads complex are then translocated into nucleus where
they associate and cooperate with DNA binding transcriptional
factors to regulate the expression of TGF-β target genes ( 8).
However, Smad transcription factors have low affinity to DNA
and need to interact with transcriptional cofactors such as
Snail and ZEB factors (see below) to achieve high affinity and
selectivity for target genes. TGF-β receptors, Smad3 and Smad4
are all essential for TGF-β-induced EMT, as suppressing the
expression of those genes by dominant negative forms blocks
TGF-β-induced EMT ( 9). In addition, TGF-β also cooperates with other signaling
pathways such as Wnt ( 10, 11), Hedgehog ( 12), Notch ( 13),
Ras-MAPK ( 14) to induce EMT.
Transcriptional regulation of EMT
The loss of epithelial markers and acquisition of mesenchymal
markers are typical feature of EMT. Among these, loss of
E-cadherin is considered as a hallmarker of EMT. E-cadherin
is a calcium dependent glycoprotein constituting the major
transmembrane component of adherens junctions, and is
responsible for epithelial cell-cell adhesion and maintenance
of cytoskeleton organization. Loss of function of E-cadherin
is thought to contribute to progression of cancer by
increasing proliferation, invasion and metastasis. A number of
transcriptional factors have been identified as transcriptional
repressor of E-cadherin during EMT such as Snail, ZEB,
and bHLH family factors like Twist, KLF8 and FoxC2. They
suppress the transcription of E-cadherin through binding the
E-box sequence containing a core 5’-CACCTG-3’ motif within
its promoter. Based on their effects on E-cadherin promoter,
Thiery JP et al. ( 3) classified these transcriptional repressors
into two groups : Snail1, Snail2, ZEB1/δEF1, SIP1/ZEB2, E47
and KLF8 directly bind and repress the activity of E-cadherin,
whereas transcriptional factors such as Twist, Goosecoid, E2.2,
and FoxC2 repress E-cadherin transcription indirectly. Among
these transcriptional repressors, Snail1 is the first repressor
identified to regulate the transcription of E-cadherin and
promote EMT. In response to signal from EMT inducer like
TGF- β, Snail factors are induced with the cooperation of smads
and HMGA2 ( 15, 16). In addition, the TGF- β pathway can also
cooperate with Ras, Notch and Wnt signaling to induce Snail
expression. Furthermore, Snail is also induced by other grow
factor such as EGF, HGF and FGF via Ras-MAPK or PI3KAKT
pathway. Upon activation, Snail1 and other transcriptional
factors such as Smads complex can bind to the E-box consensus
sequences in E-cadherin promoter, recruit the transcriptional
cofactor such as mSIN3A, HDAC1 and HDAC2 ( 17, 18) to
modify the chromatin structure, leading to the transcriptional repression of E-cadherin. Additionally, Snail factors not only
regulate expression of E-cadherin, but also modify the epithelial
and mesenchymal phenotype. For example, Snail1 was shown to
repress the expression of claudin-3,-4 and -7 ( 19, 20), which are
major components of tight junctions. Snail proteins also activate
the mesenchymal proteins such as fibronectin ( 17, 21) and
N-cadherin ( 22). Similarly, ZEB factors are also upregulated in response to
TGF-β or other growth factors ( 23). ZEB proteins then interact
with Smad3 and repress the expression of epithelial marker genes
during EMT ( 24). In addition, epigenetic alteration is also involved in the
regulation of E-cadherin, for example, hypermethylation in the
promoter region results in the loss of E-cadherin expression,
associated with EMT phenotype in breast cancer ( 25).
miRNA and EMT
miRNAs are single-stranded, 18-24nt non-coding RNA
molecules that regulate gene expression at the posttranscriptional
level through binding to 3’UTRs of target
mRNAs, usually resulting in gene silencing. Recently several
miRNAs have been identified to regulate EMT in development
and cancer. miR-200 family members (miRNA-141,miRNA-
200a, b and c, miR-429) suppress EMT mainly through targeting
the transcriptional activator of EMT, ZEB1 and ZEB2 ( 26, 27).
Interestingly, ZEB1 and ZEB2 have also been shown to suppress
the expression of miR-200 family members through binding to
E-box in the promoter of miR-200 family member, suggesting
that miR-200 members and ZEB factors reciprocally control
each other in a double negative feedback loop ( 28).
|
|
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300.[LinkOut]
- van ZN. Neoadjuvant strategies for non-small cell lung cancer, Lung Cancer 2001;34:s145-50.[LinkOut]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871-90.[LinkOut]
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One 2008;3:e2888.[LinkOut]
- Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol 2007;213:374-83.[LinkOut]
- Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res 2006;12:5369-76.[LinkOut]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelialmesenchymal transitions. Nat Rev Mol Cell Biol 2006;7:131-42.[LinkOut]
- Fuxe J, VincentT, de Herreros AG. Transcriptional crosstalk between TGFbeta and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle 2010 Jun 12;9(12). [Epub ahead of print][LinkOut]
- Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial -mesenchymal cell transition. Mol Biol Cell 2005;16:1987-2002.[LinkOut]
- Shin SY, Rath O, Zebisch A, Choo SM, Kolch W, Cho KH. Functional roles of multiple feedback loops in extracellular signal-regulated kinase and Wnt signaling pathways that regulate epithelial-mesenchymal transition. Cancer Res 2010;70:6715-24.[LinkOut]
- Eger A, Stockinger A, Park J, Langkopf E, Mikula M, Gotzmann J, et al. beta-Catenin and TGFbeta signalling cooperate to maintain a mesenchymal phenotype after FosER-induced epithelial to mesenchymal transition. Oncogene 2004;23:2672-80.[LinkOut]
- Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 2004;431:707-12.[LinkOut]
- Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev 2004;18:99-115.[LinkOut]
- Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia 2004;6:603-10.[LinkOut]
- Thuault S, Tan EJ, Peinado H, Cano A, Heldin CH, Moustakas A. HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. J Biol Chem 2008;283:33437-46.[LinkOut]
- Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, et al. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol 2009;11:943-50.[LinkOut]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelialmesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000;2:76-83.[LinkOut]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000;2:84-9.[LinkOut]
- De CB, Gilbert B, Stove C, Bruyneel E, van RF, Berx G. The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res 2005;65:6237-44.[LinkOut]
- Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci 2003;116:1959-67.[LinkOut]
- Olmeda D, Jorda M, Peinado H, Fabra A, Cano A. Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene 2007;26:1862-74.[LinkOut]
- Moreno-Bueno G, Cubillo E, Sarrio D, Peinado H, Rodriguez-Pinilla SM, Villa S, et al. Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial-mesenchymal transition, Cancer Res 2006;66:9543-56.[LinkOut]
- Shirakihara T, Saitoh M, Miyazono K. Differential regulation of epithelial and mesenchymal markers by deltaEF1 proteins in epithelial mesenchymal transition induced by TGF-beta. Mol Biol Cell 2007;18:3533-44.[LinkOut]
- Postigo AA, Depp JL, Taylor JJ, Kroll KL. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J 2003;22:2453-62.[LinkOut]
- Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci U S A 1995;92:7416-9.[LinkOut]
- Ceppi P, Mudduluru G, Kumarswamy R, Rapa I, Scagliotti GV, Papotti M, et al. Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in non-small cell lung cancer. Mol Cancer Res 2010;8:1207-16.[LinkOut]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008;10:593-601.[LinkOut]
- Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res 2008;68:7846-54.[LinkOut]
- Teschendorff AE, Journee M, Absil PA, Sepulchre R, Caldas C. Elucidating the altered transcriptional programs in breast cancer using independent component analysis. PLoS Comput Biol 2007;3:e161.[LinkOut]
- Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res 2007;13:7003-11.[LinkOut]
- Prudkin L, Liu DD, Ozburn NC, Sun M, Behrens C, Tang X, et al. Epithelial-to-mesenchymal transition in the development and progression of adenocarcinoma and squamous cell carcinoma of the lung. Mod Pathol 2009;22:668-78.[LinkOut]
- Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res 2009;69:2400-7.[LinkOut]
- Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, et al. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol 2007;31:277-83.[LinkOut]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39.[LinkOut]
- Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 2004;305:1163-7.[LinkOut]
- Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res 2008;14:2895-9.[LinkOut]
- Thomson S, Buck E, Petti F, Griffin G, Brown E, Ramnarine N, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of nonsmall-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res 2005;65:9455-62.[LinkOut]
- Rho JK, Choi YJ, Lee JK, Ryoo BY, Na II, Yang SH, et al. Epithelial to mesenchymal transition derived from repeated exposure to gefitinib determines the sensitivity to EGFR inhibitors in A549, a non-small cell lung cancer cell line. Lung Cancer 2009;63:219-26.[LinkOut]
- Yao Z, Fenoglio S, Gao DC, Camiolo M, Stiles B, Lindsted T, et al. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci U S A 2010;107:15535-40.[LinkOut]
- Yauch RL, Januario T, Eberhard DA, Cavet G, Zhu W, Fu L, et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res 2005;11:8686-98.[LinkOut]
- Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells 2009;27:2059-68.[LinkOut]
- Zhuo W, Wang Y, Zhuo X, Zhang Y, Ao X, Chen Z. Knockdown of Snail, a novel zinc finger transcription factor, via RNA interference increases A549 cell sensitivity to cisplatin via JNK/mitochondrial pathway. Lung Cancer 2008;62:8-14.[LinkOut]
- Zhuo WL, Wang Y, Zhuo XL, Zhang YS, Chen ZT. Short interfering RNA directed against TWIST, a novel zinc finger transcription factor, increases A549 cell sensitivity to cisplatin via MAPK/mitochondrial pathway. Biochem Biophys Res Commun 2008;369:1098-102.[LinkOut]
- Li QQ, Chen ZQ, Cao XX, Xu JD, Xu JW, Chen YY, et al. Involvement of NF-kappaB/miR-448 regulatory feedback loop in chemotherapy-induced epithelial-mesenchymal transition of breast cancer cells. Cell Death Differ 2010 Aug 27. [Epub ahead of print].[LinkOut]
- Li HL, Xie SM, Zhang L, Cai CJ, Wang W, Huang J, et al. Establishment and characterization of a new drug surviving cell line Am1010, derived directly from muscle metastases of a human lung adenocarcinoma patient with multi-drug-resistance to cisplatin, taxol, and gefitinib. Acta Pharmacol Sin 2010;31:601-8.[LinkOut]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature 2008;456:593-8.[LinkOut]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704-15.[LinkOut]
- Al-Hajj M, Wicha MS, ito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983-8.[LinkOut]
- Battula VL, Evans KW, Hollier BG, Shi Y, Marini FC, Ayyanan A, et al. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells 2010;28:1435-45.[LinkOut]
- Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemnessinhibiting microRNAs. Nat Cell Biol 2009;11:1487-95.[LinkOut]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823-35.[LinkOut]
- Liang Y, Zhong Z, Huang Y, Deng W, Cao J, Tsao G, et al. Stem-like cancer cells are inducible by increasing genomic instability in cancer cells. J Biol Chem 2010;285:4931-40.[LinkOut]
- Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 2009;15:232-9.[LinkOut]
- Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A 2009;106:13820-5.[LinkOut]
Cite this article as: Xiao DK, He JX. Epithelial mesenchymal transition and lung cancer. J Thorac Dis 2010;2(3):154-159. doi: 10.3978/j.issn.2072-1439.2010.02.03.7
|