Rac1 is a potential target to circumvent radioresistance
Radiation therapy is a main treatment for many types of cancer that uses high-energy rays to destroy cancer cells. The most types of radiation commonly used for cancer treatment include X-rays, gamma rays and charged particles. Ionizing radiation (IR) induces DNA damage, resulting in either cell-cycle arrest or apoptosis (1,2), to show anti-tumor activity, and is used alone or in combination with surgery, chemotherapy or immunotherapy to benefit cancer patients at different stages (3). For example, radiation therapy is used for breast cancer of earlier stage following breast-conserving surgery. It is also recommended after mastectomy for most patients with breast tumors larger than 5 cm or with metastasis to lymph nodes (4). Although IR showed positive therapeutic efficacy for many cancers, development of radioresistance, resulting in the tumor recurrence and metastasis involved multiple signaling pathways, remains a substantial clinical problem (5,6). The gene expressions affected by radiation therapy have been reported to be associated with anti-apoptosis, tumor aggressiveness, and enhanced metastatic potential such as resistance to hypoxia, invasiveness and motility, and epithelial-to-mesenchymal transition (EMT) (2). However, the critical regulator, which is involved in these processes and could be a potential target to circumvent radioresistance, has not been documented yet.
As illustrated in Figure 1, Yan and colleagues, reported RAC1 (Ras-related C3 botulinum toxin substrate 1) as a potential target to reduce radioresistance in breast cancer cells (7) as well as pancreatic cancer cells (8). Rac1, a member of Rho family GTPase, functions as a binary molecular switch by cycling between an inactive GDP-bound and an active GTP-bound state. It transduces signals from receptor tyrosine kinase (RTK), G-protein-couple receptors (GPCRs), integrins and stress to control a number of essential cellular functions including motility, adhesion, and proliferation. Rac1 GTPase is overexpressed or hyperactivated in various types of tumors and implicates in tumorigenesis, angiogenesis, invasion and metastasis (9). It has been repeatedly reported that Rac1 can be activated by IR treatment in some types of tumor cells, including breast cancer (10), pancreatic cancer (8), and head and neck squamous cell carcinoma (7) and is involved in the formation of radioresistance. In 2012, Yan and colleagues reported that IR induced G2/M checkpoint arrest through activation of ATM/ATR pathway and inhibition of Cdc25 phosphatase in breast cancers, but accompanied with the activation of ERK1/2 signaling, suggesting a potential survival signal contributing the development of radioresistance. In their recently study published in Oncogene, Rac1 expression and activity were increased in the hyper-fractionated radiation (HFR)-selected breast cancer cells (7). Furthermore, inhibition of Rac1 by using pharmacological inhibitor NSC23766 and dominant negative mutant of RAC1 was found to abolish MEK/ERK signaling and downregulate anti-apoptotic protein Bcl-xL/MCL1L expressions to increase the sensitivity of HFR-selected breast cancer cells to irradiation. In addition to activating pro-survival pathways, Rac1 is also involved in cell invasion and metastasis of breast cancer by regulating actin cytoskeletal structure (11). IR has been reported to process a pro-metastatic trans-effect, which rests on the endothelial radiation response promoting the extravasation of circulating tumor cells, in a RAC1-dependent manner (12). Inhibition of RAC1 by NSC23766 in vascular endothelial cells also repressed the adhesion to endothelial cells and the extravasation of colon carcinoma cells in vivo, revealing that RAC1 in both tumor cells and endothelial cells contributes to tumor progression and metastasis in response to irradiation.
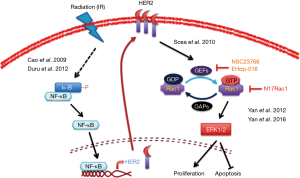
Although accumulating evidences indicate Rac1 as a key mediator in controlling breast cancer cell recurrence in response to irradiation treatment, it remains an open question how IR induces RAC1 activation or expression. Short-term treatment with IR increases the active GTP-bound state of RAC1 without affecting its protein level in breast (7), pancreatic (8), and head and neck squamous cell carcinoma (HNSCC) (13). Both RAC1 and phosphatidylinositol 3,4,5-trisphosphate-dependent Rac guanine nucleotide exchange factor-1 (P-Rex1), highly overexpressed in breast cancers, can be activated by GPCR CXCR4 or ErbB signaling pathways to contribute to breast cancer cells progression and proliferation and their insensitization to antiestrogen therapy (14,15). It has been demonstrated that human epidermal growth factor receptor 2 (HER2) expression was induced by radiation to mediate radioresistance in breast cancer cells with a low basal level of HER2 (HER2-/low) (16). Moreover, NF-κB activation plays an essential role in up-regulating radiation-induced HER2 expression (17). These findings suggest that radiation activates Rac-GEFs/Rac1 signaling via inducing the NF-κB-dependent HER2 expression signaling and thus leads to radioresistance in breast cancer cells. In supporting to this notion, combined treatment with ErbB tyrosine kinase inhibitor and irradiation showed synergistic anti-tumor activity (18). Similarly, RAC1 has also been viewed as a critical regulator for cancer stem cells activity of non-small cell lung cancer (19). Furthermore, EGFR pathway has also been reported to activate Rac1 through inducing accumulation of T-cell lymphoma invasion and metastasis 1 (Tiam1), one Rac1-GEFs, in non-small-cell lung cancer and colon cancer to promote their invasion, migration and metastasis (20), solidifying the critical role of ErbB/Rac signaling pathway in various cancer type in response to irradiation treatment and chemotherapy. Interestingly, suppression of HMG-CoA reductase by lovastatin also attenuated IR-induced metastasis via downregulating E-selectin (12), suggesting a role of HMG-CoA reductase in activation of RAC1 via production of lipid for anchoring RAC1 at plasma membrane. However, further studies are required for this speculation.
Acknowledgements
None.
Footnote
Provenance: This is an invited Commentary commissioned by the Section Editor Hongcheng Zhu (Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Maier P, Hartmann L, Wenz F, et al. Cellular pathways in response to ionizing radiation and their targetability for tumor radiosensitization. Int J Mol Sci 2016;17:E102. [Crossref] [PubMed]
- Martin OA, Anderson RL, Narayan K, et al. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis? Nat Rev Clin Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Baskar R, Lee KA, Yeo R, et al. Cancer and radiation therapy: current advances and future directions. Int J Med Sci 2012;9:193-9. [Crossref] [PubMed]
- Cianfrocca M, Gradishar WJ. Controversies in the therapy of early stage breast cancer. Oncologist 2005;10:766-79. [Crossref] [PubMed]
- Menaa C, Li JJ. The role of radiotherapy-resistant stem cells in breast cancer recurrence. Breast Cancer Manag 2013;2:89-92. [Crossref] [PubMed]
- Ahmad A. Pathways to breast cancer recurrence. ISRN Oncol 2013;2013:290568.
- Hein AL, Post CM, Sheinin YM, et al. RAC1 GTPase promotes the survival of breast cancer cells in response to hyper-fractionated radiation treatment. Oncogene 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Yan Y, Hein AL, Etekpo A, et al. Inhibition of RAC1 GTPase sensitizes pancreatic cancer cells to γ-irradiation. Oncotarget 2014;5:10251-70. [Crossref] [PubMed]
- Rathinam R, Berrier A, Alahari SK. Role of Rho GTPases and their regulators in cancer progression. Front Biosci (Landmark Ed) 2011;16:2561-71. [Crossref] [PubMed]
- Yan Y, Greer PM, Cao PT, et al. RAC1 GTPase plays an important role in γ-irradiation induced G2/M checkpoint activation. Breast Cancer Res 2012;14:R60. [Crossref] [PubMed]
- Baugher PJ, Krishnamoorthy L, Price JE, et al. Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phenotype of human breast cancer cells. Breast Cancer Res 2005;7:R965-74. [Crossref] [PubMed]
- Hamalukic M, Huelsenbeck J, Schad A, et al. Rac1-regulated endothelial radiation response stimulates extravasation and metastasis that can be blocked by HMG-CoA reductase inhibitors. PLoS One 2011;6:e26413. [Crossref] [PubMed]
- Skvortsov S, Dudás J, Eichberger P, et al. Rac1 as a potential therapeutic target for chemo-radioresistant head and neck squamous cell carcinomas (HNSCC). Br J Cancer 2014;110:2677-87. [Crossref] [PubMed]
- Sosa MS, Lopez-Haber C, Yang C, et al. Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol Cell 2010;40:877-92. [Crossref] [PubMed]
- Yang C, Klein EA, Assoian RK, et al. Heregulin beta1 promotes breast cancer cell proliferation through Rac/ERK-dependent induction of cyclin D1 and p21Cip1. Biochem J 2008;410:167-75. [Crossref] [PubMed]
- Duru N, Fan M, Candas D, et al. HER2-associated radioresistance of breast cancer stem cells isolated from HER2-negative breast cancer cells. Clin Cancer Res 2012;18:6634-47. [Crossref] [PubMed]
- Cao N, Li S, Wang Z, et al. NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance. Radiat Res 2009;171:9-21. [Crossref] [PubMed]
- Gurtner K, Ebert N, Pfitzmann D, et al. Effect of combined irradiation and EGFR/Erb-B inhibition with BIBW 2992 on proliferation and tumour cure in cell lines and xenografts. Radiat Oncol 2014;9:261. [Crossref] [PubMed]
- Akunuru S, Palumbo J, Zhai QJ, et al. Rac1 targeting suppresses human non-small cell lung adenocarcinoma cancer stem cell activity. PLoS One 2011;6:e16951. [Crossref] [PubMed]
- Zhu G, Fan Z, Ding M, et al. An EGFR/PI3K/AKT axis promotes accumulation of the Rac1-GEF Tiam1 that is critical in EGFR-driven tumorigenesis. Oncogene 2015;34:5971-82. [Crossref] [PubMed]


