Glucose-insulin-potassium correlates with hemodynamic improvement in patients with septic myocardial dysfunction
Introduction
Septic shock is the most common cause of death in intensive care units with a mortality rate as high as 40–60% (1). Much of the associated mortality and morbidity is due to refractory hypotension and cardiovascular collapse. Catecholamines such as norepinephrine, epinephrine, and dopamine are effective in maintaining mean arterial pressure (MAP), but may also decrease cardiac output, oxygen delivery, and blood flow to vital organs (2).
Glucose-insulin-potassium (GIK) has been shown to improve myocardial perfusion and left ventricular function by providing metabolic support and preventing ischemia-related metabolic abnormalities (3-6). Although a number of clinical trials on GIK use in patients with acute coronary syndrome has shown variable results (7,8), a recent study revealed a decrease in the composite outcome of cardiac arrest or mortality and infarct size in patients administered GIK early in out-of-hospital acute coronary syndrome (9).
One of the manifestations of cardiovascular dysfunction in septic shock is septic myocardial depression. It is usually a reversible phenomenon caused by myocardial depressant factors and inefficient metabolism rather than myocardial hypoperfusion (10). The cardioprotective effects of GIK may be beneficial in this situation and are primarily via insulin, resulting in more efficient myocardial metabolism and an anti-inflammatory effect. Several studies have reported the use of GIK in septic myocardial depression (11-13); however, the mechanism of GIK in improving hemodynamics remained unclear. We hypothesized that septic shock with myocardial depression would differ from one without, and the cardioprotective action of insulin may improve hemodynamics in septic shock with myocardial depression. The aim of this study was to (I) compare the baseline characteristics and changes of hemodynamic outcomes; and (II) examine the relationship between insulin and hemodynamic outcomes in GIK-treated patients with or without septic myocardial depression.
Methods
Study design and data collection
In our institution, a GIK protocol was employed in patients with severe sepsis/septic shock (14), heart failure, and other inflammatory conditions between October 2012 and March 2014. In this retrospective cohort study, patients with severe sepsis or septic shock that were admitted to the intensive care unit with refractory shock and treated with GIK were included. Patients were divided into hypodynamic and non-hypodynamic septic shock groups according to echocardiographic findings. Hypodynamic septic shock was characterized by decreased heart function and MAP despite a positive fluid balance and use of vasopressors (12,13). In this study, heart function was measured with echocardiograms rather than with invasive hemodynamic monitoring, as invasive hemodynamic monitoring is not routinely used for these patients in current practice (15). Baseline demographic and clinical characteristics were collected and included demographic factors, comorbidities, causes of sepsis, vital signs, laboratory data before GIK infusion, types of vasopressors, and infusion rates. The study outcomes included: serial changes of MAP and the heart rate (HR) during the 72-h study period; correlation between the insulin dose in GIK and improvement in MAP and the cardiovascular Sequential Organ Failure Assessment (SOFA) score (16) at 72 h compared with baseline (Δ MAP and cardiovascular Δ SOFA, respectively); success rate of weaning of the vasopressor; vasopressor-free days; ventilator-free days; and mortality. To assess the safety of GIK infusions, we determined any possible GIK-related adverse events and reviewed serum concentrations of glucose and potassium at baseline, day 2, and day 3 of treatment. The study protocol was approved by the institutional review board of the Asan Medical Center (No. 2015-0092) and informed consent required from each patient was waived due to the retrospective nature of the study.
Patient exclusion criteria
The exclusion criteria were as follows: irreversible state such as fulminant hepatic failure with no planned liver transplantation; do not resuscitate order; death within 24 h of receiving GIK infusion; concurrent heart failure (New York Heart Association class III or IV) with shock; or cases in which the efficacy of GIK infusion was difficult to determine such as GIK infusion more than 72 h after the onset of shock.
GIK infusion protocol
Therapy with GIK was initiated in patients with severe septic shock who required high-dose vasopressor therapy such as norepinephrine (≥0.2 µg/kg/min) and vasopressin (≥0.01 U/min) despite adequate fluid resuscitation. The intravenous GIK solution consisted of 30% glucose (300 g/L), 50 U/L of regular insulin, and 80 mEq/L of KCl/L (9). The protocol was to administer GIK intravenously at 1.5 mL/kg/h for the first 72 h and maintain infusion at 40 mL/h until disease improvement or death of the patient. In patients with acute kidney injury (F-failure, L-loss of kidney function, or E-end-stage renal failure of RIFLE criteria) (17) or liver cirrhosis, GIK was administered at 40 mL/h for the first 24 h, and if tolerated, the infusion rate was increased to 1.5 mL/kg/h. Serum glucose was measured every 2 h, and an additional regular insulin infusion was maintained at 150–180 mg/dL. Serum potassium was measured as needed to maintain a concentration of 3.5–5.5 mEq/L. If available, an echocardiogram was used to identify and follow the progression of septic myocardial depression (18).
Definitions
An immunosuppressive condition was diagnosed if there was an underlying disease that affected the immune system such as hematologic malignancy or if immunosuppressive therapy was being administered (e.g., chemotherapy, radiotherapy, steroid, and other immunosuppressants). Empirical therapy was considered to have been appropriate if at least one effective antimicrobial was included in the initial antibiotic therapy. The severity of illness was assessed by the Acute Physiology and Chronic Health Evaluation II score (19) and SOFA score, excluding the neurology component from the SOFA score as it was difficult to analyze during anesthesia and/or sedation (16,20). The inotropic score was calculated as [dopamine dose (µg/kg/min)] + [dobutamine dose (µg/kg/min)] + [100× epinephrine dose (µg/kg/min)] + [100× norepinephrine dose (µg/kg/min)] + [100× phenylephrine dose (µg/kg/min)] (21). The vasopressor dependency index was calculated as the ratio of inotropic score to MAP (22). Success of vasopressor weaning was defined as the ability of the patient to maintain normal pressure for 48 h without any vasopressor support. Echocardiographic findings of septic myocardial dysfunction included left ventricular, right ventricular, or biventricular dilation and depression (18).
Statistical analysis
Continuous variables are presented as medians and interquartile ranges (IQRs), and were compared with the Mann-Whitney U test. Categorical variables are presented as percentages of patients and were compared by the chi-square or Fisher’s exact test. For multiple comparisons, the areas under the curve relative to baseline values for continuous variables with repeated measurements were calculated and compared between the two groups with the Mann-Whitney U test as previously described (23). The linear relationship between two variables was assessed by Spearman’s correlation coefficient. All tests of significance reported were two tailed, and a P value less than 0.05 was considered significant. Statistical analyses were performed using SPSS version 22.0K for Windows (SPSS Inc., Chicago, IL, USA).
Results
During the study period, 63 patients fulfilled the inclusion criteria. Following exclusion of 18 patients, there were 45 septic shock patients remaining that underwent GIK treatment. Prior to GIK infusion, an echocardiogram was performed in 33 of 45 (73%) patients. Hypodynamic septic shock was identified in 12 patients with an echocardiographic finding of septic myocardial depression (see supplementary appendix online for further details, Table S1), and non-hypodynamic septic shock was identified in 16 patients with a normal echocardiogram. Five patients were excluded from the analysis due to chronic cardiac dysfunction (Figure 1).

Full table
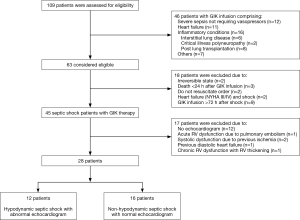
Baseline characteristics
Baseline characteristics of the study patients are shown in Table 1. Patients in the non-hypodynamic were older and comprised more males than in the hypodynamic group, although this was not statistically significant. There were no differences between the two groups with regard to comorbidities, with the exception that immunosuppressive conditions were more frequently observed in patients in the hypodynamic group than in patients in the non-hypodynamic group (75% vs. 44%, respectively; P=0.10). Septic shock was primarily due to pneumonia in 42% (5/12) of patients in the hypodynamic group and 50% (8/16) of patients in the non-hypodynamic group (P=0.66). There was no significant difference between the groups in regard to the isolation of Gram-negative and Gram-positive bacteria. Appropriate antibiotic therapy was administered to all patients in both groups. The median (IQR) MAP was 69 [60–70] mmHg for the hypodynamic group and 78 [70–88] mmHg for the non-hypodynamic group (P=0.08). The median (IQR) HR was 129 [117–138]/min for the hypodynamic group and 95 [82–104]/min for the non-hypodynamic group (P<0.001). There were no significant differences between the two groups with regard to other vital signs, laboratory data, and severity scores. Fluid therapy and mechanical ventilation was not found to differ between the hypodynamic group and the non-hypodynamic group.
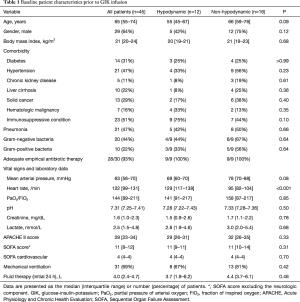
Full table
Baseline vasopressor requirements of the study patients are depicted in Table 2. In both groups, all patients required norepinephrine prior to GIK infusion with a median rate of 0.29 µg/kg/min in both groups (P=0.76). Vasopressin and hydrocortisone were administered to approximately half of the patients in each group. The median (IQR) inotropic score and the median (IQR) vasopressor dependency index were similar between the two groups [34.0 (18.0–57.0) vs. 29.0 (18.0–47.0), P=0.69; and 5.0 (2.4–8.2) vs. 3.7 (2.3–6.4), P=0.49, respectively].
Full table
Changes of hemodynamic outcomes
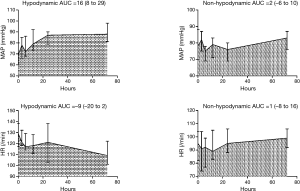
In 45 patients, the median (IQR) duration from the onset of shock to GIK administration was 14 [7–22] h, and the median (IQR) duration of GIK infusion was 70 [36–130] h. The median (IQR) total insulin dose at 72 h was 142 [97–351] U/L. The changes of hemodynamic outcomes during the 72-h study period are shown in Figure 2. MAP significantly increased in the hypodynamic group with the median (IQR) area under the curve of 16 (8 to 29) mmHg compared with 2 (−6 to 10) mmHg in the non-hypodynamic group (P=0.003). Conversely, HR decreased in the hypodynamic group with the median (IQR) area under the curve of −9 (−20 to 2)/min compared with 1 (−8 to 16)/min in the non-hypodynamic group, although this did not reach statistical significance (P=0.13).
Insulin: relation with hemodynamics
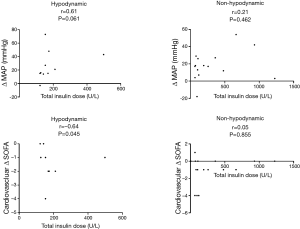
Figure 3 shows the correlation between the total insulin dose and hemodynamic outcomes. Total insulin dose was observed to correlate with an improvement in MAP at 72 h (Δ MAP) (r=0.61, P=0.061) and in the cardiovascular SOFA score at 72 h (cardiovascular Δ SOFA) (r=−0.64, P=0.045) in the hypodynamic group but not in the non-hypodynamic group.
Other outcomes
The secondary outcomes of the study group are described in Table 3. There were no significant differences between the two groups in regard to weaning outcomes and mortality.

Full table
Safety assessment
Table 4 shows serum glucose and potassium monitoring of the study patients. A higher baseline serum glucose was observed in the hypodynamic group than in the non-hypodynamic group, although this was not statistically significant. During the 72-h study period, the median serum glucose was observed to gradually decrease in both groups. A significant increase in the median (IQR) serum potassium from 4.1 (3.6–4.4) mEq/L on day 2 to 4.6 (3.7–5.1) mEq/L on day 3 was found in the non-hypodynamic group, although serum potassium levels were within the normal range (≤5.5 mEq/L).

Full table
Discussion
The present study showed that GIK increased MAP and tended to decrease HR in the hypodynamic group, compared with the non-hypodynamic group. Also, the insulin dose of GIK was closely related to hemodynamic outcomes in patients in the hypodynamic group. These results may explain the possible role of GIK in septic myocardial depression. GIK was well tolerated by all study patients, with minimal adverse drug reactions that did not mandate discontinuation. To our knowledge, this study is the first to evaluate the effects of GIK infusion in patients with septic shock.
In addition to myocardial ischemia, myocardial depression is also an important feature in septic shock. Myocardial depressant factors such as TNF-α, IL-1β, and nitric oxide (24-26) and ineffective metabolism by increased free fatty acids (27) may contribute to septic myocardial depression. Insulin appears to suppress the secretion and antagonize the harmful effects of TNF-α, macrophage migration inhibitory factor, and superoxide anion (28-30) and increase anti-inflammatory signal transduction (31-33). Furthermore, insulin has been found to suppress free fatty acids and increase the utilization of glucose, providing an efficient energy source (34,35). Due to the anti-inflammatory and metabolic effects of insulin, in combination with providing glucose to the myocardium, GIK appears to be a useful approach in septic shock patients with myocardial depression.
Experimental studies have been performed to support the use of GIK in septic shock, although the primary mechanism through insulin improves cardiac performance in septic shock is inconsistent among studies (36,37). Several clinical studies have addressed the use of GIK in patients with septic myocardial depression (11-13). However, in these studies, the effects of GIK in improving hemodynamics have not been fully evaluated. The beneficial effects of GIK therapy have been demonstrated to be greater when insulin was administered at a high dose during reperfusion (38,39). In the present study, the total insulin dose in GIK correlated with the improvement of MAP and cardiovascular SOFA scores in the hypodynamic group, but not in the non-hypodynamic group. The findings presented here suggest that GIK may be effective in septic shock exacerbated by a reduced cardiac output such as septic myocardial depression, and that the dosage of insulin may be important in this situation. Previous GIK studies in septic shock (11-13) have shown a temporary reduction in vasopressor infusion with short-term GIK infusion (within 30 minutes in two of three studies), and a mortality rate as high as 73–100% has been reported. In this study, the median duration of GIK infusion was approximately 3 days, and the percentage of patients that survived to hospital discharge was 67% in the hypodynamic group (non-hypodynamic group: 44%). Despite recent advances in the treatment of sepsis and septic shock, these preliminary findings are of great interest, and further studies are required to define the optimal duration of GIK therapy in septic shock.
The present study has several limitations. First, this study was a descriptive analysis of GIK therapy in septic shock patients without a control group. It is likely that the response to standard therapy for septic shock, such as fluid resuscitation, appropriate antibiotic therapy, and early infection control (15), may have partially resulted in an improvement in hemodynamics or other outcomes. Second, a substantial proportion of patients (12/45, 27%) were excluded from the primary analysis because of the lack of an echocardiogram. Inclusion of these patients may have influenced the outcomes of both patient groups in this study. Third, GIK therapy may have been less effective in the present study due to the time delay in GIK infusion (median duration from the onset of shock to GIK infusion, 14 h) and relatively low insulin dose (median total insulin dose at 72 h, 142 U/L). Hence, our results cannot be considered conclusive. Prospective controlled studies with standardized protocols of GIK therapy including dosage and timing are required.
In conclusion, the present study demonstrated that GIK therapy was related to short-term hemodynamic improvement in septic shock patients with myocardial depression. The use of GIK was well tolerated with minimal adverse drug reactions. Further studies are required to demonstrate the role of GIK in septic myocardial dysfunction.
Acknowledgements
We thank Nicola Edwards and Sundrela Kamhieh-Milz who provided language editing services (Bioedit Ltd).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional review board of the Asan Medical Center (No. 2015-0092) and informed consent required from each patient was waived due to the retrospective nature of the study.
References
- Annane D, Aegerter P, Jars-Guincestre MC, et al. Current epidemiology of septic shock: the CUB-Réa Network. Am J Respir Crit Care Med 2003;168:165-72. [Crossref] [PubMed]
- Bomzon L, Rosendorff C. Renovascular resistance and noradrenaline. Am J Physiol 1975;229:1649-53. [PubMed]
- Sodi-Pallares D, Testelli MR, Fishleder BL, et al. Effects of an intravenous infusion of a potassium-glucose-insulin solution on the electrocardiographic signs of myocardial infarction. A preliminary clinical report. Am J Cardiol 1962;9:166-81. [Crossref] [PubMed]
- Gupta DK, Jewitt DE, Young R, et al. Increased plasma-free-fatty-acid concentrations and their significance in patients with acute myocardial infarction. Lancet 1969;2:1209-13. [Crossref] [PubMed]
- Maroko PR, Libby P, Sobel BE, et al. Effect of glucose-insulin-potassium infusion on myocardial infarction following experimental coronary artery occlusion. Circulation 1972;45:1160-75. [Crossref] [PubMed]
- Apstein CS, Gravino FN, Haudenschild CC. Determinants of a protective effect of glucose and insulin on the ischemic myocardium. Effects on contractile function, diastolic compliance, metabolism, and ultrastructure during ischemia and reperfusion. Circ Res 1983;52:515-26. [Crossref] [PubMed]
- Malmberg K, Rydén L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995;26:57-65. [Crossref] [PubMed]
- Mehta SR, Yusuf S, Díaz R, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA 2005;293:437-46. [Crossref] [PubMed]
- Selker HP, Beshansky JR, Sheehan PR, et al. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: the IMMEDIATE randomized controlled trial. JAMA 2012;307:1925-33. [Crossref] [PubMed]
- Krishnagopalan S, Kumar A, Parrillo JE, et al. Myocardial dysfunction in the patient with sepsis. Curr Opin Crit Care 2002;8:376-88. [Crossref] [PubMed]
- Bronsveld W, van den Bos GC, Thijs LG. Use of glucose-insulin-potassium (GIK) in human septic shock. Crit Care Med 1985;13:566-70. [Crossref] [PubMed]
- Mauritz W, Schindler I, Zadrobilek E, et al. Glucose-potassium-insulin in hypodynamic septic shock. Anaesthesist 1986;35:623-7. [PubMed]
- Hamdulay SS, Al-Khafaji A, Montgomery H. Glucose-insulin and potassium infusions in septic shock. Chest 2006;129:800-4. [Crossref] [PubMed]
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250-6. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. [Crossref] [PubMed]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204-12. [Crossref] [PubMed]
- Griffee MJ, Merkel MJ, Wei KS. The role of echocardiography in hemodynamic assessment of septic shock. Crit Care Clin 2010;26:365-82. table of contents. [Crossref] [PubMed]
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. [Crossref] [PubMed]
- Payen DM, Guilhot J, Launey Y, et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med 2015;41:975-84. [Crossref] [PubMed]
- Wernovsky G, Wypij D, Jonas RA, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 1995;92:2226-35. [Crossref] [PubMed]
- Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA 2009;301:2445-52. [Crossref] [PubMed]
- Morelli A, Ertmer C, Westphal M, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA 2013;310:1683-91. [Crossref] [PubMed]
- Cain BS, Meldrum DR, Dinarello CA, et al. Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Crit Care Med 1999;27:1309-18. [Crossref] [PubMed]
- Kumar A, Thota V, Dee L, et al. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med 1996;183:949-58. [Crossref] [PubMed]
- Kumar A, Brar R, Wang P, et al. Role of nitric oxide and cGMP in human septic serum-induced depression of cardiac myocyte contractility. Am J Physiol 1999;276:R265-76. [PubMed]
- Mjos OD. Effect of free fatty acids on myocardial function and oxygen consumption in intact dogs. J Clin Invest 1971;50:1386-9. [Crossref] [PubMed]
- Das UN. Possible beneficial action(s) of glucose-insulin-potassium regimen in acute myocardial infarction and inflammatory conditions: a hypothesis. Diabetologia 2000;43:1081-2. [Crossref] [PubMed]
- Das UN. Newer uses of glucose-insulin-potassium regimen. Med Sci Monit 2000;6:1053-5. [PubMed]
- Das UN. Is insulin an antiinflammatory molecule? Nutrition 2001;17:409-13. [Crossref] [PubMed]
- Das UN. Insulin and the critically ill. Crit Care 2002;6:262-3. [Crossref] [PubMed]
- Das UN. Insulin and inflammation: further evidence and discussion. Nutrition 2002;18:526-7. [Crossref] [PubMed]
- Li J, Zhang H, Wu F, et al. Insulin inhibits tumor necrosis factor-alpha induction in myocardial ischemia/reperfusion: role of Akt and endothelial nitric oxide synthase phosphorylation. Crit Care Med 2008;36:1551-8. [Crossref] [PubMed]
- McNulty PH. Comparison of local and systemic effects of insulin on myocardial glucose extraction in ischemic heart disease. Am J Physiol Heart Circ Physiol 2000;278:H741-7. [PubMed]
- Wallhaus TR, Taylor M, DeGrado TR, et al. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation 2001;103:2441-6. [Crossref] [PubMed]
- Holger JS, Dries DJ, Barringer KW, et al. Cardiovascular and metabolic effects of high-dose insulin in a porcine septic shock model. Acad Emerg Med 2010;17:429-35. [Crossref] [PubMed]
- Levenbrown Y, Penfil S, Rodriguez E, et al. Use of insulin to decrease septic shock-induced myocardial depression in a porcine model. Inflammation 2013;36:1494-502. [Crossref] [PubMed]
- Doenst T, Bothe W, Beyersdorf F. Therapy with insulin in cardiac surgery: controversies and possible solutions. Ann Thorac Surg 2003;75:S721-8. [Crossref] [PubMed]
- Zhang L, Zhang L, Li YH, et al. High-dose glucose-insulin-potassium treatment reduces myocardial apoptosis in patients with acute myocardial infarction. Eur J Clin Invest 2005;35:164-70. [Crossref] [PubMed]


