One-stage hybrid surgery for acute Stanford type A aortic dissection with David operation, aortic arch debranching, and endovascular graft: a case report
Introduction
Considering its extremely complex surgical strategy and high risk of complications, acute Stanford type A aortic dissection involves ascending aorta, aortic arch, and descending aorta and remains a challenge for cardiovascular surgeons (1). The dissection of Stanford type A aortic involves aortic valve dysfunction, total aortic repair, and aortic valve surgery and is a high-risk operation with high mortality rate and cerebral complications, especially for those with high age and surgical risks (2). Recently, with the development of endovascular interventions, a one-stage hybrid operation that combines open surgical and endovascular techniques is widely used to avoid deep hypothermic circulatory arrest and cardiopulmonary bypass (CPB) time; this technique gained excellent outcomes (3,4). Bentall operation and its modifications are the “golden standard” for the treatment of combined ascending aorta and aortic valve conditions; however, if the aortic valve is less compromised, then the valve-sparing aortic operations, also known as David procedures, can be used as better alternatives (5). We report a complex case of one-stage hybrid surgery that combines David procedure, aortic arch debranching, and endovascular aortic repair. This surgery is performed on a patient with acute Stanford type A aortic dissection and severe aortic regurgitation.
Case presentation
A 59-year-old woman was admitted to the emergency department because of a sudden onset of thoracic and abdominal pain. Medical history revealed 8 years of poorly controlled hypertension, chronic obstructive pulmonary disease, and neurologic stroke 2 years before the admission. The vitals were stable; upon admission, the blood pressure was 136/67 mmHg, and the heart rate was 88 bpm (sinus rhythm). Emergent computed tomography angiography (CTA) diagnosed a Stanford type A aortic dissection that extends from the aortic root to the bilateral external iliac artery and involves the brachiocephalic trunk, celiac trunk, and left renal artery (Figure 1A). Transesophageal echocardiography (TEE) confirmed the diagnosis with an enlarged aortic root and ascending aorta (50 mm) and moderate to severe aortic regurgitation (Figure 1B).
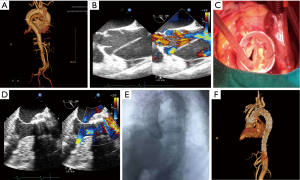
The extremely high surgical risk for traditional total arch repairs with deep hypothermic arrest was considered. The patient underwent emergent one-stage hybrid procedure that combines David operation, aortic arch debranching, and endovascular aortic repair. After a standard median sternotomy and exposure of the heart and great vessels, ultrasound-guided aortic arch cannulation and a standard right atrium cannulation were performed to establish the CPB (6). After aortic clamping and cardioplegia, ascending aorta was incised from the bottom to the opening of brachiocephalic trunk. The native aortic valve was carefully reviewed, and the diameter of the annulus was measured, followed by the isolation of the coronary ostia as buttons. The aortic sinuses were resected up to a rim of 4–5 mm of the aortic wall, and the commissures were lifted by suturing lines. The diameter of the sinu-tubular junction was measured, and a 30-mm graft was used. Therefore, the remnants of aortic sinuses and the aortic annulus were sutured inside the graft. The commissures were pulled up and also sutured inside the graft. Isolated coronary ostia were re-implanted to their respective neo-sinuses (Figure 1C). Another 30-mm four-branched graft was used to connect the 30-mm graft to the distal aorta, after which the aorta was re-opened. The brachiocephalic trunk, left carotid artery, and left subclavian artery were separately anatomized to the prosthetic branch. CPB was then terminated, and TEE found an acceptable systolic function and traces of aortic regurgitation (Figure 1D).
The transfemoral approach was used for the endovascular procedure. Under the guidance of angiography, the two intravascular stent grafts were implanted to cover all the aortic arches, starting in the descending aorta from the distal anastomotic point in the ascending aorta until the level of celiac trunk. Further angiogram confirmed that all dissecting areas were covered, and all three supra-aortic branch grafts were well-functioning without endoleakage (Figure 1E). The patient was transferred to the intensive care unit after surgery. The postoperative course was uneventful. Post-operative CTA and echocardiography revealed the patency of the three-branched vessels and the optimal position of the graft (Figure 1F). The patient was discharged on the 12th post-operative day. During the 3 months of follow-up, the patient did not complain of any unpleasant feeling, and the echocardiography confirmed the healthy condition of the heart and large vessels.
Discussion
Acute Stanford type A aortic dissection is a lethal pathological condition with extremely high mortality rate even after surgical repair (7). Conventional open repair is associated with substantial morbidity and mortality, especially for elderly patients (4). Recently, with the improved outcomes of thoracic endovascular aortic repair, the hybrid surgery approach for acute Stanford type A aortic dissection treatment was recognized as less invasive for patients (8). Using deep hypothermic risk, this hybrid procedure combines open interventions for ascending aorta and aortic arch with ante-grade (or retrograde); endovascular graft became the frontline treatment option for many complex aortic pathologies of patients at high risk of traditional total arch replacement surgery (9). In the present case, we performed a novel one-stage hybrid surgery for a patient with acute Stanford A aortic dissection. The patient underwent a successful David operation, aortic disbranching, and endovascular graft through femoral access. Although open surgical repair is the standard treatment for type A aortic dissection, this method has high in-hospital mortality rate (10). In addition, this procedure presents a high frequency of transient or permanent neurological and cognitive dysfunctions, ranging between 3% and 17% (11,12). In contrast, hybrid surgery significantly reduced the mortality and morbidity because deep hypothermic circulatory arrest and decreased CPB time were avoided; therefore, the hybrid surgery is a feasible alternative treatment in high risk patients (4,13).
For this type of hybrid procedure, the management of supra-aortic vessels is important according to the aortic arch involvement. The surgical strategy of the aortic arch and supra-aortic vessels depends on the dissection of supra-aortic vessels dissect and the distance of the proximal landing zone (14). A traditional total arch replacement presents high-risk surgery and high incidence of severe complications as a result of the long circulatory arrest (15). The 30-day mortality of the hemiarch replacement with concomitant TEVAR is 26%, and the temporary neurological dysfunction and permanent stroke rates are 20% and 7%, respectively (16). In contrast, the 30-day mortality decreased to 11.9% with a stroke rate 7.6% for the debranching procedures with TEVAR; this procedure is less invasive because circulatory arrest significantly reduced CPB (17) or aortic cross-clamping is not required. Therefore, debranching procedures for type A dissection have unique advantages.
Preserving the native aortic valve potentially benefits the physiological valve function and does not require life-long anticoagulation (18). The indication of David procedure is the pathology of the ascending aorta with aortic insufficiency when the pre-operative examination showed that the undamaged aortic valve is free of sclerosis or calcification. The surgeon makes the final decision after carefully inspecting the aortic valve (5). Although Shrestha et al. reported the excellent long-term outcomes of David procedures and further concluded its suitability as an elective and isolated procedure in non-connective tissue disease, the use of David procedures in acute Stanford type A aortic dissection is still controversial (5). Beckmann et al. conducted an interesting clinical study that compares Bentall and David procedures. The David group presented the lowest incidences of both 30-day and long-term mortality and the highest rate of valve-related reoperations. In addition, the authors recommended that David procedures should be considered especially in younger patients and should be performed only by surgeons familiar with the technique (19). Consistent with these results, the David procedures in the present study did not compromise the survival compared with Bentall procedures and even significantly improved the longevity of the survival (20).
Conclusions
In summary, the patient underwent one-stage hybrid surgery that combines David procedures, debranching, and trans-femoral endovascular graft. The one-stage surgery successfully reduced the trauma and surgery time. Our experience provided novel and well-designed combined techniques for treating acute Stanford type A aortic dissection. Most importantly, our techniques significantly lowered the risks, thereby expanding the indications of surgical intervention for acute Stanford type A aortic dissection. However, our findings need further studies to provide additional information that can help cardiovascular surgeons handle complex acute Stanford type A aortic dissection.
Acknowledgements
Funding: This study is supported, in part, by Grant No. 81200144 from the National Research Foundation of Nature Science, China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. This case report was approved by the hospital ethics committee.
References
- Ma WG, Zhu JM, Zheng J, et al. Sun's procedure for complex aortic arch repair: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg 2013;2:642-8. [PubMed]
- Chou HT, Lo JP, Chua CH, et al. Initial Experience of Modified Four-Branched Graft Technique and Antegrade TEVAR in Acute Type A Aortic Dissection. Ann Thorac Cardiovasc Surg 2015;21:481-6. [Crossref] [PubMed]
- Sun L, Qi R, Zhu J, et al. Repair of acute type A dissection: our experiences and results. Ann Thorac Surg 2011;91:1147-52. [Crossref] [PubMed]
- Antoniou GA, Mireskandari M, Bicknell CD, et al. Hybrid repair of the aortic arch in patients with extensive aortic disease. Eur J Vasc Endovasc Surg 2010;40:715-21. [Crossref] [PubMed]
- Shrestha M, Baraki H, Maeding I, et al. Long-term results after aortic valve-sparing operation (David I). Eur J Cardiothorac Surg 2012;41:56-61; discussion 61-2. [PubMed]
- Noiseux N, Couture P, Sheridan P, et al. Aortic cannulation for type A dissection: guidance by transesophageal echocardiography. Interact Cardiovasc Thorac Surg 2003;2:178-80. [Crossref] [PubMed]
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Tanaka A, Sandhu HK, Estrera AL. Descending endografts for type A dissections: con. Ann Cardiothorac Surg 2016;5:227-32. [Crossref] [PubMed]
- Marullo AG, Bichi S, Pennetta RA, et al. Hybrid aortic arch debranching with staged endovascular completion in DeBakey type I aortic dissection. Ann Thorac Surg 2010;90:1847-53. [Crossref] [PubMed]
- Moon MC, Morales JP, Greenberg RK. The aortic arch and ascending aorta: are they within the endovascular realm? Semin Vasc Surg 2007;20:97-107. [Crossref] [PubMed]
- Sundt TM 3rd, Orszulak TA, Cook DJ, et al. Improving results of open arch replacement. Ann Thorac Surg 2008;86:787-96; discussion 787-96. [Crossref] [PubMed]
- Strauch JT, Böhme Y, Franke UF, et al. Selective cerebral perfusion via right axillary artery direct cannulation for aortic arch surgery. Thorac Cardiovasc Surg 2005;53:334-40. [Crossref] [PubMed]
- Liu P, Chang Q, Qian X, et al. Early and mid-term results after hybrid total arch repair of DeBakey type I dissection without deep hypothermic circulatory arrest. Interact Cardiovasc Thorac Surg 2016;23:608-15. [Crossref] [PubMed]
- Higashi R, Matsumura Y, Yamaki F. A single stage hybrid repair of a complicated acute type B dissection with aortic arch involvement. Ann Vasc Dis 2014;7:141-4. [Crossref] [PubMed]
- Poon SS, Theologou T, Harrington D, et al. Hemiarch versus total aortic arch replacement in acute type A dissection: a systematic review and meta-analysis. Ann Cardiothorac Surg 2016;5:156-73. [Crossref] [PubMed]
- Vallabhajosyula P, Gottret JP, Robb JD, et al. Hemiarch replacement with concomitant antegrade stent grafting of the descending thoracic aorta versus total arch replacement for treatment of acute DeBakey I aortic dissection with arch tear†. Eur J Cardiothorac Surg 2016;49:1256-61; discussion 1261. [Crossref] [PubMed]
- Moulakakis KG, Mylonas SN, Markatis F, et al. A systematic review and meta-analysis of hybrid aortic arch replacement. Ann Cardiothorac Surg 2013;2:247-60. [PubMed]
- Nishida H, Tabata M, Fukui T, et al. Surgical Strategy and Outcome for Aortic Root in Patients Undergoing Repair of Acute Type A Aortic Dissection. Ann Thorac Surg 2016;101:1464-9. [Crossref] [PubMed]
- Beckmann E, Martens A, Alhadi FA, et al. Is Bentall Procedure Still the Gold Standard for Acute Aortic Dissection with Aortic Root Involvement? Thorac Cardiovasc Surg 2016;64:116-23. [PubMed]
- Subramanian S, Leontyev S, Borger MA, et al. Valve-sparing root reconstruction does not compromise survival in acute type A aortic dissection. Ann Thorac Surg 2012;94:1230-4. [Crossref] [PubMed]


