Veno-venous extracorporeal membrane oxygenation: cannulation techniques
Introduction
The development of extracorporeal membrane oxygenation (ECMO) technology allows a new approach for the intensive care management of acute cardiac and/or respiratory failure in adult patients who are not responsive to conventional treatment (1). Current ECMO therapies provide a variety of options for the multidisciplinary teams who are involved in the management of these critically ill patients (2). Veno-venous ECMO (VV-ECMO) can provide quite complete respiratory support, even if this highly complex technique presents substantial risks, such as bleeding, thromboembolic events and infection (3). While VV-ECMO circuits usually include the cannulation of two vessels (double cannulation) in the classic configuration, the use of a single cannula is now possible for VV-ECMO support. Recently, expert centers have proposed the use of more advanced approaches, such as the cannulation of three vessels (triple cannulation), which follows veno-arterio-venous (VAV) or veno-arterio-pulmonary-arterial cannulation (VAPa), for use in specific situations. While the first configuration corresponds to a very useful method to ensure both circulatory and respiratory support, the second, even if still experimental, aims to provide additional support in the case of right heart failure during VAV-ECMO (4). Nevertheless, ‘triple’ cannulation expands the field of application but enhances the complexity of ECMO systems. The purpose of the present article is to review the indications for VV-ECMO, patient assessment prior to cannulation, the role of ultrasound-guided vessel puncture, double lumen single bicaval cannulations and finally triple cannulation in VV-ECMO.
Indications for VV-ECMO
VV-ECMO is indicated in patients with severe respiratory failure that is refractory to optimal mechanical ventilation and medical therapy (5). ECMO for respiratory failure in adults is usually managed with a VV configuration, i.e., blood is drained from the right atrium (RA) or superior vena cava (SVC) and inferior vena cava (IVC) and returned into the RA (6). This technique places the artificial lung in series with the normal lungs rather than in parallel (as in cardiopulmonary bypass). The oxygenated blood mixes with the native venous return (which did not pass through the ECMO circuit), such that the resultant arterial PaO2 and saturation represents a mixture of the oxygenated extracorporeal blood and the unoxygenated venous blood that passes through the nonfunctional native lungs. This desaturated arterial blood, combined with the normal cardiac output (CO), provides more than adequate systemic oxygen delivery to support metabolism, and the airway is managed at rest settings. With a VV configuration, the patient relies on his own hemodynamics, such that CO and pulmonary and systemic vascular resistances are unchanged during extracorporeal gas exchange.
The main indications for which VV-ECMO should be considered as a treatment option are reversible respiratory failure including acute respiratory distress syndrome (ARDS) either due to bronchopulmonary aspiration, bacterial, viral or atypical pneumonia, barotrauma or acute or chronic interstitial pneumonitis (6,7). Patients with advanced and/or irreversible diseases, such as uncontrolled sepsis, non-pulmonary multi-organ failure, irreversible neurological injury, terminal illness or other life-limiting disease should not be candidates for VV-ECMO. Moreover, patients with chronic respiratory failure or ventilator-dependent respiratory failure who are not eligible to be bridged to lung transplantation should not be considered as candidates for VV-ECMO (8,9). Finally, patients with associated cardiac dysfunction and/or cardiogenic shock should receive veno-arterial (VA) ECMO support (10).
Patient assessment prior to cannulation
Candidates for VV-ECMO are typically severely hypoxemic and/or hypercapnic and unresponsive to optimal medical management, including protective ventilation with low-tidal volumes (11) and plateau pressure less than 28–30 cmH2O (12), high levels of PEEP (13), prone positioning (14), neuromuscular blockers (15) and/or other adjunctive therapies, including nitrous oxide (16) or almitrine (17). The recent literature suggests that a PaO2/FIO2 ratio of 70–80 mmHg, Murray score >3, and pH <7.2 provide a reasonable threshold for considering VV-ECMO in adults with ARDS (18,19). It is crucial to determine the acute nature of the pulmonary failure, exclude cardiac and/or other organ failure and verify that the respiratory failure cannot be improved with optimal ventilator management.
In the case of VV-ECMO indications, it is imperative to identify any patient characteristics that may prevent VV-ECMO implantation. Thus, before VV-ECMO insertion, a comprehensive echocardiographic examination should be performed, as permitted by the patient’s hemodynamic condition. The use of VV-ECMO support depends on the underlying etiology of respiratory failure. Whereas the incidence of right ventricular (RV) failure has been considerably reduced by the lung protective ventilation strategy in severe ARDS patients, it still remains as high as 25% (20,21). Even if respiratory failure is predominant, the choice of ECMO configuration that would best support the patient is not always simple. VV-ECMO helps resolve hypoxemia and hypercapnia, allowing lower plateau pressures and resulting in reduced pulmonary vascular resistance. This may improve the hemodynamic instability associated with RV failure. Nevertheless, the etiologies of RV failure may not be immediately reversible, and it may be difficult to determine what proportion of the hemodynamic instability can be attributed to the underlying metabolic disturbances. The presence of significant concomitant left ventricular (LV) dysfunction requires the use of VA-ECMO. In this setting, echocardiography plays an essential role in evaluating the degree of residual LV function.
From a theoretical standpoint, a thorough examination of the vascular anatomy will assist in determining any potential barriers to cannulation. The femoral and internal jugular veins (IJVs) should be assessed by echography to detect underlying vascular disease, such as deep venous thrombosis, or the presence of a caval filter that may preclude cannula placement. The size of the venous drainage cannula is a determining factor for blood flow in the ECMO circuit; therefore, the insertion in the largest cannula should be attempted. The diameter of the vessels measured by ultrasound may aid in the choice of cannula size (22). From a practical standpoint, this anatomical evaluation is rarely performed in daily clinical practice before VV-ECMO implantation considering the critical state of these patients. The insertion of cannulae could be technically challenging in morbidly obese patients. Finally, supplementary information, such as coagulopathy or any contraindications to anticoagulation, should be stated.
Role of ultrasound-guided vessel puncture
Ultrasound has a definite role in achieving vascular access for difficult-to-cannulate patients, and growing evidence suggests that routine utilization of ultrasound guidance increases the success rate and decreases complications in all vascular applications. Cannulation of the IJV with real-time ultrasound guidance is now a standard practice that is highly recommended by many societies and supported by a vast amount of evidence (23,24). Recent studies have shown that ultrasound guidance increases the safety of alternative central venous routes as well as femoral access (25,26). Thus, growing evidence suggests that routine utilization of ultrasound guidance is helpful for all types of vascular access. However, some clinical situations where the ultrasound technique is not feasible require the use of the traditional anatomical landmarks technique (27) and/or fluoroscopic guidance. Indeed, even if VV-ECMO cannulation is usually performed in the ICU under ultrasound guidance alone, it can be carried out in a cardiac catheterization laboratory or a classical operating room using C-arm fluoroscopy or, more recently, in a hybrid operating room combining both imaging tools. In these settings, the use of fluoroscopy ensures proper positioning of the wires and the cannula (10). However, the presence of a skilled operator and the use of proper technique are required to achieve success and avoid complications (28).
Double VV-ECMO cannulation
The percutaneous approach is typically the technique of choice in VV-ECMO. Even if the placement of VV-ECMO cannulae is essentially performed in the ICU, this can be performed in a variety of hospital settings: an emergency room, a cardiac catheterization laboratory or an operating room. A recent retrospective study showed that percutaneous cannulation for VV-ECMO can be achieved with a high degree of success and low complication rate by physicians using ultrasound and/or fluoroscopic guidance (29). However, the procedure is preferably performed in the ICU with skilled teams where all the required equipment is promptly available (10).
Before initiating the procedure, the patient needs to be deeply sedated as well as paralyzed, and the airway must be correctly secured. Moreover, a central venous line (in general in the left IJV) and an arterial catheter should be inserted, allowing an adequate hemodynamic monitoring as well as infusion of volume fluids and/or vasopressors required during the procedure. If a central venous catheter is already inserted into the right IJV and utilized as central venous line, another central venous catheter should be inserted elsewhere because the reinjection cannula should be positioned in the right IJV. In particular situations, a reinjection cannula could be placed in the right IJV proximal to the entry site of the existing central venous catheter. Echocardiography should be available at the bedside. Even if transthoracic echocardiography (TTE) sometimes allows adequate visualization of the cardiac structures, transesophageal echocardiography (TEE) is highly recommended to guide VV-ECMO cannulation when performed in the ICU and in the absence of fluoroscopy. The probe should be positioned before skin preparation. TEE has the advantage of determining the exact position of the cannulae, although the presence of artifacts may be misleading (30). It also allows for prompt diagnosis of any cannulation complications, such as pericardial effusion or cardiac tamponade.
The entire procedure should ideally be performed by two trained operators in order to insert and control the wires and introduce the cannulae. The patient is prepared and draped sterilely. Both groins should be prepared in case of puncture and/or cannulation failure or for possible conversion to VA ECMO. Current, albeit limited, evidence does not support the routine use of prophylactic antibiotics in patients receiving ECMO support (31).
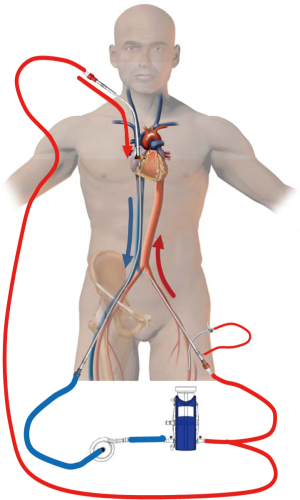
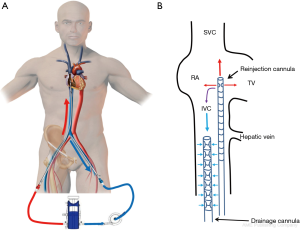
In the classic configuration, VV-ECMO support may be achieved with two cannulae, one usually inserted in the right femoral vein and advanced to the junction between the IVC and the RA and the other inserted in the right IJV and advanced through the SVC into the RA (Figure 1A). The amount of the drainage of the venous cannula directly determines blood flow. The largest possible venous cannula should be used to maximize flow and easily achieve target output.
The right IJV puncture should be percutaneously performed under ultrasound guidance. The puncture site in the neck should be 4–5 cm away from the clavicle. TEE guidance helps to guide the progression of the IJV guidewire during VV-ECMO implantation. In fact, the mid-esophageal bicaval and modified bicaval views provide excellent visualization of the IVC, SVC, tricuspid valve (TV) and RA (32). The tip of the IJV wire should be positioned at least beyond the hepatic veins to prevent displacement or migration of the wire. The guidewire should be seen in both venae cava to confirm that it has not passed through the TV and into the right ventricle, across an atrial septal defect or into the coronary sinus. During the repeated dilatation of the skin and subcutaneous tissue and while threading the cannula over the guidewire, the visualization of the guidewire allows the identification of any secondary migration (33). Attention should also be paid to monitoring any new or expanding pericardial collection (34). Occasionally, the existence of an outsized Eustachian valve at the caval-atrial junction induces a translation of the wire into the right ventricle instead of the IVC. In this case, a stiffer guidewire may be cautiously used instead of the classic flexible wire. Most of the available insertion kits contain a flexible j-tip wire and serial dilators (usually from 12 to 20 Fr).
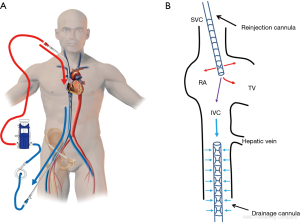
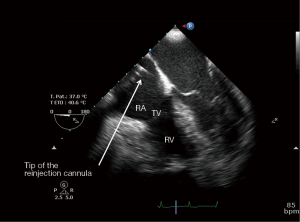
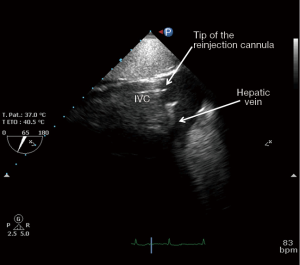
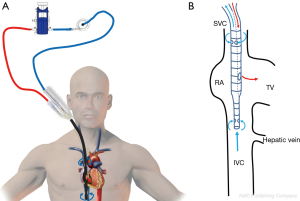
After the vascular puncture and the insertion of the guidewire using the Seldinger technique, successive dilators are placed over the guidewire to progressively enlarge the access site. According to the density of the skin and/or fascia along the wire’s pathway, forceful manipulation of the dilators may be required until the access site is large enough to allow the placement of the cannula. During each dilator exchange, the access site should be held by the second operator to reduce the blood loss. Finally, using TTE, TEE or fluoroscopic guidance, the tip of the reinjection cannula is ideally positioned in front of the TV into the RA to ensure an optimal reinjection flow and to reduce the recirculation (Figure 1B) (35). The position of the cannula is confirmed by TEE (Figure 2).
The femoral vein is then percutaneously punctured using the same technique. The right femoral vein is preferred due to a better angle with the IVC than the left vein. Following Seldinger’s technique, a long flexible j-tip guidewire (150 cm) is advanced from the femoral vein into the IVC directed toward the RA. Once again, TTE or TEE should be used to properly visualize the guidewire. To avoid dislodging or migration of the guidewire and incorrect migration of the cannula into the right ventricle or to the hepatic or renal vein, the tip of the wire should be placed in the SVC and maintained in it during the entire procedure. To obtain optimal drainage, the cannula tip should be located at the IVC-RA junction (Figure 3). If the venous cannula is not advanced far enough, there is an increased risk of the tip to aspirate against the wall of the IVC. If it is too far into the RA, there is a risk of damage to structures. It will also increase the possibility of recirculation if the venous drainage cannula is too close to the reinjection cannula (36).
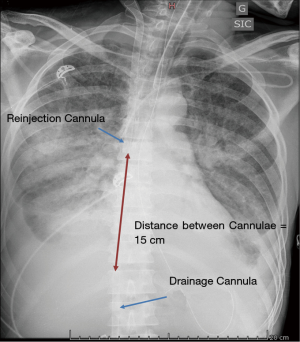
Recirculation, a phenomenon in which reinjected oxygenated blood is withdrawn by the drainage venous cannula without passing through the systemic circulation, decreases the effectiveness of VV-ECMO (36). The effectiveness of VV-ECMO in supporting oxygenation depends on several factors, including the blood flow circulating through the device, the patient’s CO and metabolic demand, the oxygen fraction in the sweep gas, the diffusion properties and surface area of the membrane and the amount of recirculation within the circuit (37,38). This conventional femoral-to-internal jugular “double” vein cannulation results itself in recirculation because the reinjection flow is directed toward the drainage port. The proximity of the reinfusion and drainage ports will, consequently, have a direct effect on the amount of recirculation, with a higher percentage of recirculated blood flow when the two ports are in closer proximity. Similarly, the orientation of the cannulae induced by the patient’s position, such as movement from a supine to a seated position or rotation of the head and neck, may affect the amount of recirculation. Fifteen centimeters between the two cannulae are usually required to decrease recirculation, while larger drainage cannulae may allow for comparable blood flow rates at lower pump speeds with less negative venous pressure, possibly diminishing the amount of recirculation (Figure 4).
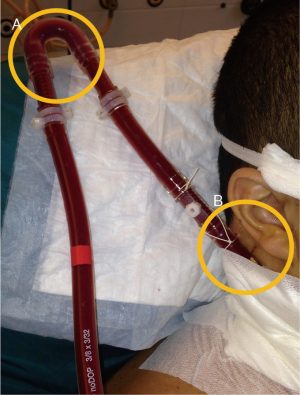
After correct placement, the cannulae can be firmly fixed in order to avoid accidental displacement. The jugular cannula can be attached to the mastoid behind the ear. The bend of the original ECMO circuit can be used to prevent kinking of the manifold (Figure 5). A chest X-ray should be performed at the end of the procedure to detect potential complications of puncture (hemothorax and/or pneumothorax) and to further confirm the correct placement of the cannulae and the distance between them.
When the cannulation of the IJV is technically not possible, an alternative configuration of VV-ECMO support involves bilateral femoral cannulation. The tip of the drainage venous cannula is placed in the IVC while the tip of the reinjection cannula is positioned into the RA (Figure 6A). This technique presents a major drawback in terms of recirculation, as the tip of the reinjection cannula cannot be properly placed in front of the TV (Figure 6B). Even if many centers use this VV-ECMO configuration, the authors recommend it as a second-line strategy when femoro-jugular cannulation cannot be performed.
Double lumen single bicaval VV-ECMO cannulation
The double lumen bicaval (DLB) cannula consists of a single cannula conceived to be inserted from the right IJV through the RA into the IVC. Considering the stiffness and the profile of this cannula, other cannulation sites, such as the left IJV or the subclavian vein, are not advocated. The DLB cannula consists of three ports: proximal, middle and distal. The proximal and the distal ports drain the deoxygenated blood from the upper and lower body, respectively, while the middle port delivers the oxygenated blood from the ECMO circuit. The cornerstone of the optimal performance of this DLB cannula consists in its good positioning. In fact, the distal port, which is situated at the tip of the cannula, should be positioned in the IVC, just below the junction between the RA and the IVC while the proximal port of the cannula should be located in the SVC (Figure 7A). The middle port of the DLB cannula should be positioned in the RA and oriented ideally in front of the TV (Figure 7B). Therefore, the oxygenated blood released by the middle port of the DLB cannula is directly ejected into the right ventricle and then into the pulmonary vessels by native cardiac function to oxygenate the blood in systemic circulation (39,40). The unique design of this DLB cannula allows minimal recirculation and the shunt of oxygenated blood (41). The other advantage of the DLB cannula is that it avoids additional femoral cannulation. This allows the use of prone positioning that is currently a common practice implemented in VV-ECMO patients to improve lung recruitment and oxygenation (42) and a patient’s early mobilization, such as sitting up in bed and even ambulating, while on VV-ECMO support (43). Ambulatory ECMO programs have been developed in an attempt to provide rehabilitation, physical therapy and minimization of sedation prior to lung transplantation to improve both surgical and post-transplant outcomes. Favorable outcomes have been reported using this novel approach, but how and where this strategy should be implemented remain unclear.
The size of the cannula should be selected prior the procedure. Manufacturer pressure/flow curves may help to choose the optimal cannula. A formula based on body surface area ×2.2 allows to estimate the optimal blood flow obtainable with the VV-ECMO support (10). However, for optimal oxygenation, the VV-ECMO flow should reach at least 60% of the CO of the patient. CO should then be measured or at least estimated by TTE, TEE or any thermodilution technique before the implantation (44). Moreover, due to strong changes in venous return or to inflammation, for example, CO may change significantly. Therefore, a system permitting continuous CO measurement should be used even after ECMO implantation. Echocardiographic and/or pulse contour methods can be used in these patients (30).
The DLB cannulae that are currently available offer limited maximal blood flow (45). Typically, the 23 Fr cannula allows a maximum flow of 3 L/min, the 27 Fr cannula is limited to 4.5 L/min, and the 31 Fr DLB cannula allows not more than 5 L/min. In these cases, hemolysis could occur if high flow rates are applied (45). Thus, it is strongly recommended to employ the largest cannula tolerated by the patient’s anatomy, or, if the 60% of the estimated patient’s CO cannot be achieved by the chosen cannula, the classic double femoro-jugular cannulation should be performed.
The puncture of the right IJV should be performed in the same way as for the femoro-jugular VV-ECMO configuration. Particular attention should be paid concerning the insertion kit, which should include serial dilators up to 24 Fr to ensure an optimal dilation if the 27 or the 31 Fr cannulae are used. Due to the stiffness, large size and length of the DLB cannula, TTE or TEE should absolutely confirm the position of the wire at each step of the procedure to avoid displacement or migration of the guidewire and a wrong migration of the cannula into the right ventricle.
Finally, TTE or TEE should confirm that the cannula tip is placed in the IVC and that the middle port of the catheter is positioned in the RA with the outflow injecting into the TV (46). Afterward, the wire is removed entirely, and the two extremities of the cannula (inflow and outflow tracts) are clamped. The ECMO circuit is then connected with the ends of the cannula while excluding air bubbles from the tubing. After the ECMO circuit is connected with both the arterial and venous parts of the cannula, the clamps are removed, the circuit is opened, and the ECMO is started until full flow. The DLB cannula is lastly tightened to the skin with several sutures. TTE or TTE should be performed to ensure that the DLB is still correctly positioned and a chest X-ray allows excluding any complication and assesses the right position of the cannula.
Triple cannulation in VV-ECMO
Triple cannulation in VV-ECMO represents a new approach for mechanical support that is set up by adding a third cannula to an existing VV-ECMO circuit. They may be used in the VAV configuration (Figure 8). Even if triple cannulation is a promising method for selected patients in experienced centers, evidence from the available literature is limited.
In some patients, traditional VV-ECMO approaches are not sufficient to both oxygenate and assist the hemodynamic state. Patients treated initially with VV-ECMO for respiratory failure may subsequently develop cardiogenic shock, requiring VA ECMO support. A typical example could be RV failure in a patient with pulmonary fibrosis associated with pulmonary hypertension. Conversely, patients equipped with VA-ECMO could develop deep hypoxemia of the upper-body, mainly concerning the heart and the brain. In such cases, a combined approach with a triple cannulation may address the problem. VAV-ECMO in ARDS patients undergoing ECMO support granted a survival benefit compared with VV-ECMO and VA-ECMO configurations alone (47). In addition, cerebral hypoxemia in patients with cardiogenic shock on VA-ECMO was successfully treated after switching to a VAV-ECMO approach (48). A recent study shows also that VAV-ECMO appears to be an effective therapeutic option for patients with respiratory failure and subsequent hemodynamic instability. This strategy could optimize ECMO therapy for patients with both cardiac and pulmonary failure (4).
Upon switching to VAV-ECMO, an additional arterial cannula is inserted for inflow in the femoral artery. This additional inflow cannula is connected to the existing arterial line using a Y connector. An arterial catheter is systematically placed distally to the entry site of the arterial cannula to prevent lower limb ischemia. The flow in both return cannulae, which also depends on cannula sizes and diameters, should be monitored with a flow sensor and adapted using a modifiable clamp. This is mandatory, as the demand of oxygenated blood flow on each return cannula differs from patient to patient and over time. Each variation in flow balance affects preload, afterload and oxygen saturations simultaneously. Variations of oxygenator and sweep gas settings will also change oxygen saturation and carbon dioxide in both return cannulae simultaneously. Hence, during VAV-ECMO, frequent echocardiography assessment and continuous upper- and lower-body oxygenation measurements must be performed to monitor right and LV filling and function, as well as tissue oxygenation. Although the respiratory support provided by VAV-ECMO is usually sufficient for lung protective ventilation, hemodynamic assistance is always lower compared to VA-ECMO (4).
VAPa is a special alternative type of VAV-ECMO. This configuration has not been yet validated in studies. While VAV-ECMO associates the features of VV and VA-ECMO, VAPa-ECMO aims to provide additional support in the case of right heart failure throughout VAV-ECMO. In this configuration, the returning venous cannula is advanced throughout the TV, the right ventricle, and the pulmonary valve into the pulmonary artery. For VPa cannulation, a flexible cannula is recommended. Transesophageal echocardiographic or fluoroscopic guidance cannulation is highly recommended. As with VAV-ECMO, the flow balance should be managed with a clamp and a flow sensor. Similar to VA and VPa cannulation, the tip of the drainage cannula should be located in the RA to optimize the drainage of the upper and lower body.
For each VV-ECMO configuration, systemic anticoagulation is required to avoid thrombus formation in the cannulae and in the circuit. Therefore, as the guidewire(s) is(are) checked to be correctly positioned, a 5,000 unit intravenous bolus of heparin is usually administered prior to the introduction of the cannula (10,49). In the present manuscript, readers may evaluate all ECMO configurations, cannula sizes, cannula lengths, respective tip positions, blood flows supported, the amount of recirculation, potential hemodynamic support, risk of limb ischemia and cannula prices in a detailed Table 1 to estimate the costs of the technique.
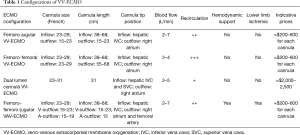
Full table
Conclusions
VV-ECMO is the treatment of choice for patients with respiratory failure refractory to optimal mechanical ventilation and conventional medical treatments. A baseline echocardiographic evaluation is, however, of paramount importance in such critically ill patients to rule out the presence of concomitant cardiac dysfunction. The femoro-jugular technique should be the first-line strategy for VV-ECMO in consideration of its ease of implantation and its effectiveness. The femoro-femoral technique should be reserved for when the cannulation of the IJV is not technically feasible. Double lumen single cannula is an appealing option for ambulatory VV-ECMO programs, but more complex implantation and higher costs have limited its widespread application. Finally, further data are necessary in order to best define the potential role of a ‘triple’ cannulation strategy in combined severe cardiopulmonary failure.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Abrams D, Brodie D. Novel Uses of Extracorporeal Membrane Oxygenation in Adults. Clin Chest Med 2015;36:373-84. [Crossref] [PubMed]
- Combes A, Brodie D, Bartlett R, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med 2014;190:488-96. [Crossref] [PubMed]
- Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014;97:610-6. [Crossref] [PubMed]
- Ius F, Sommer W, Tudorache I, et al. Veno-veno-arterial extracorporeal membrane oxygenation for respiratory failure with severe haemodynamic impairment: technique and early outcomes. Interact Cardiovasc Thorac Surg 2015;20:761-7. [Crossref] [PubMed]
- Bartlett RH, Deatrick KB. Current and future status of extracorporeal life support for respiratory failure in adults. Curr Opin Crit Care 2016;22:80-5. [Crossref] [PubMed]
- Delnoij TS, Driessen R, Sharma AS, et al. Venovenous Extracorporeal Membrane Oxygenation in Intractable Pulmonary Insufficiency: Practical Issues and Future Directions. Biomed Res Int 2016;2016:9367464.
- Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 2015;7:E166-76. [PubMed]
- Tulman DB, Stawicki SP, Whitson BA, et al. Veno-venous ECMO: a synopsis of nine key potential challenges, considerations, and controversies. BMC Anesthesiol 2014;14:65. [Crossref] [PubMed]
- Fan E, Gattinoni L, Combes A, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory failure: A clinical review from an international group of experts. Intensive Care Med 2016;42:712-24. [Crossref] [PubMed]
- Shaheen A, Tanaka D, Cavarocchi NC, et al. Veno-Venous Extracorporeal Membrane Oxygenation (V V ECMO): Indications, Preprocedural Considerations, and Technique. J Card Surg 2016;31:248-52. [Crossref] [PubMed]
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338:347-54. [Crossref] [PubMed]
- Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010;303:865-73. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Sokol J, Jacobs SE, Bohn D. Inhaled nitric oxide for acute hypoxemic respiratory failure in children and adults. Cochrane Database Syst Rev 2003.CD002787. [PubMed]
- Roch A, Papazian L, Bregeon F, et al. High or low doses of almitrine bismesylate in ARDS patients responding to inhaled NO and receiving norepinephrine? Intensive Care Med 2001;27:1737-43. [Crossref] [PubMed]
- Extracorporeal Life Support Organization. Welcome to ELSO! Available online: https://www.elso.org/
- Aokage T, Palmér K, Ichiba S, et al. Extracorporeal membrane oxygenation for acute respiratory distress syndrome. J Intensive Care 2015;3:17. [Crossref] [PubMed]
- Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med 2013;39:1725-33. [Crossref] [PubMed]
- Bouferrache K, Vieillard-Baron A. Acute respiratory distress syndrome, mechanical ventilation, and right ventricular function. Curr Opin Crit Care 2011;17:30-5. [Crossref] [PubMed]
- Conrad SA, Grier LR, Scott LK, et al. Percutaneous cannulation for extracorporeal membrane oxygenation by intensivists: a retrospective single-institution case series. Crit Care Med 2015;43:1010-5. [Crossref] [PubMed]
- Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med 2012;38:1105-17. [Crossref] [PubMed]
- Troianos CA, Hartman GS, Glas KE, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2011;24:1291-318. [Crossref] [PubMed]
- Prabhu MV, Juneja D, Gopal PB, et al. Ultrasound-guided femoral dialysis access placement: a single-center randomized trial. Clin J Am Soc Nephrol 2010;5:235-9. [Crossref] [PubMed]
- Powell JT, Mink JT, Nomura JT, et al. Ultrasound-guidance can reduce adverse events during femoral central venous cannulation. J Emerg Med 2014;46:519-24. [Crossref] [PubMed]
- Giraud R, Bendjelid K. When ultrasound-guided catheterization is useless: back to landmarks! Crit Care 2014;18:452. [Crossref] [PubMed]
- Reusz G, Csomos A. The role of ultrasound guidance for vascular access. Curr Opin Anaesthesiol 2015;28:710-6. [PubMed]
- Burns J, Cooper E, Salt G, et al. Retrospective Observational Review of Percutaneous Cannulation for Extracorporeal Membrane Oxygenation. ASAIO J 2016;62:325-8. [Crossref] [PubMed]
- Douflé G, Roscoe A, Billia F, et al. Echocardiography for adult patients supported with extracorporeal membrane oxygenation. Crit Care 2015;19:326. [Crossref] [PubMed]
- O'Horo JC, Cawcutt KA, De Moraes AG, et al. The Evidence Base for Prophylactic Antibiotics in Patients Receiving Extracorporeal Membrane Oxygenation. ASAIO J 2016;62:6-10. [Crossref] [PubMed]
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013;26:921-64. [Crossref] [PubMed]
- Yastrebov K, Manganas C, Kapalli T, et al. Right ventricular loop indicating malposition of J-wire introducer for double lumen bicaval venovenous extracorporeal membrane oxygenation (VV ECMO) cannula. Heart Lung Circ 2014;23:e4-7. [Crossref] [PubMed]
- Hirose H, Yamane K, Marhefka G, et al. Right ventricular rupture and tamponade caused by malposition of the Avalon cannula for venovenous extracorporeal membrane oxygenation. J Cardiothorac Surg 2012;7:36. [Crossref] [PubMed]
- Bonacchi M, Harmelin G, Peris A, et al. A novel strategy to improve systemic oxygenation in venovenous extracorporeal membrane oxygenation: the "χ-configuration". J Thorac Cardiovasc Surg 2011;142:1197-204. [Crossref] [PubMed]
- Abrams D, Bacchetta M, Brodie D. Recirculation in venovenous extracorporeal membrane oxygenation. ASAIO J 2015;61:115-21. [Crossref] [PubMed]
- Agerstrand CL, Bacchetta MD, Brodie D. ECMO for adult respiratory failure: current use and evolving applications. ASAIO J 2014;60:255-62. [Crossref] [PubMed]
- Schmidt M, Tachon G, Devilliers C, et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med 2013;39:838-46. [Crossref] [PubMed]
- Bermudez CA, Rocha RV, Sappington PL, et al. Initial experience with single cannulation for venovenous extracorporeal oxygenation in adults. Ann Thorac Surg 2010;90:991-5. [Crossref] [PubMed]
- Javidfar J, Brodie D, Wang D, et al. Use of bicaval dual-lumen catheter for adult venovenous extracorporeal membrane oxygenation. Ann Thorac Surg 2011;91:1763-8; discussion 1769.
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011;365:1905-14. [Crossref] [PubMed]
- Kimmoun A, Roche S, Bridey C, et al. Prolonged prone positioning under VV-ECMO is safe and improves oxygenation and respiratory compliance. Ann Intensive Care 2015;5:35. [Crossref] [PubMed]
- Garcia JP, Kon ZN, Evans C, et al. Ambulatory veno-venous extracorporeal membrane oxygenation: innovation and pitfalls. J Thorac Cardiovasc Surg 2011;142:755-61. [Crossref] [PubMed]
- Romagnoli S, Zagli G, Ricci Z, et al. Cardiac output: a central issue in patients with respiratory extracorporeal support. Perfusion 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Napp LC, Kühn C, Hoeper MM, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol 2016;105:283-96. [Crossref] [PubMed]
- Platts DG, Sedgwick JF, Burstow DJ, et al. The role of echocardiography in the management of patients supported by extracorporeal membrane oxygenation. J Am Soc Echocardiogr 2012;25:131-41. [Crossref] [PubMed]
- Stöhr F, Emmert MY, Lachat ML, et al. Extracorporeal membrane oxygenation for acute respiratory distress syndrome: is the configuration mode an important predictor for the outcome? Interact Cardiovasc Thorac Surg 2011;12:676-80. [Crossref] [PubMed]
- Moravec R, Neitzel T, Stiller M, et al. First experiences with a combined usage of veno-arterial and veno-venous ECMO in therapy-refractory cardiogenic shock patients with cerebral hypoxemia. Perfusion 2014;29:200-9. [Crossref] [PubMed]
- The Extracorporeal Life Support Organization (ELSO). ELSO Anticoagulation Guideline. 2014. Available online: https://www.elso.org/portals/0/files/elsoanticoagulationguideline8-2014-table-contents.pdf