Detection of patient-ventilator asynchrony should be improved: and then what?
Invasive mechanical ventilation often saves the lives of patients suffering from acute respiratory distress. Placement and maintenance of an endotracheal airway usually requires sedation and analgesia, which generally results in cessation of spontaneous breathing and the need for controlled mechanical ventilation. Today, modern intensive care medicine concepts aim at early reduction of the level of sedation (1) to promote spontaneous breathing efforts (2,3) and early mobilization (4), in order to avoid negative effects of deep and prolonged sedation and (respiratory) muscle weakness (5). Epidemiological data suggest an increase in the use of partial ventilator support modes (6).
Spontaneous breathing activity during time-cycled, volume preset assist control (AC) ventilation allows triggering of mandatory breaths, but the patients’ ability to interact with the ventilator is otherwise limited. In patients with a high rate of spontaneous breathing activity, inspiratory efforts outside the trigger intervals may result in a second preset breath, which immediately follows the first breath before (complete) expiration was possible (7). In volume-preset AC, such breath stacking asynchrony may not only cause discomfort for the patient, but can result in uncontrolled delivery of tidal volumes (VT) that are up to twice as high as intended (7). These high VT may promote ventilator-induced lung injury (VILI) in patients with and without acute respiratory distress syndrome (ARDS) (8). Stacked breaths often remain undetected leaving the treating physicians unaware of this potential risk.
Detection of patient ventilator asynchrony
In a recent issue of Intensive Care Medicine, Dr. Beitler and colleagues (9) describe objective criteria for quantifying breath stacking asynchrony (BREATHE criteria). They are based on an automated analysis of flow curves performed for up to 72 hours counting the following events: consecutive inspiratory ventilator cycles, expiratory volume threshold [≥2 mL/kg predicted body weight (PBW) less than prior inspiratory volume], cumulative inspiratory volume threshold (≥2 mL/kg PBW above intended VT), expiratory time threshold (<1 s), and inspiratory time threshold (≥120% of preset inspiratory time). The authors compared their new definition with a method previously described by Thille (10), which defines breath stacking asynchrony as “two [inspiratory] cycles separated by a very short expiratory time, defined as less than one-half the mean inspiratory time, the first cycle being patient-triggered” (10). In addition, Beitler and colleagues used visual inspection, performed by two experienced intensivists, for comparison. Semi-automated application of the BREATHE criteria detected more high VT breaths as compared with the Thille method (10), whereas the latter method found more desynchronized breaths. Agreement of the BREATHE method and visual inspection was at least 97%. Breath stacking occurred in about three fourth of hours during the observation time, presuming no use of neuromuscular blockade (NMB). With NMB use, breath stacking asynchrony was eliminated (9).
Although the occurrence of breath stacking had been reported previously (7,11), a major contribution of Dr. Beitler’s study is that it discusses patient-ventilator asynchrony as a phenomenon that often goes clinically undetected or underestimated and shows how to improve its detection by the BREATHE criteria.
Once detected, however, a crucial question is, how to improve patient-ventilator interaction. Two basic approaches are available for decreasing patient-ventilator asynchrony:
(I) Adaptation of the patient to the ventilator by increasing sedation/analgesia with or without muscle paralysis;
(II) Adaptation of the ventilator to the patient (ventilator adjustment or change in ventilator mode).
Adapting the patient to the ventilator
In the study by Beitler et al., the use of NMB was shown to abolish breath stacking asynchrony effectively (9). The somewhat outdated concept of NMB use in patients with early ARDS was rediscovered by recent data showing improved 90 days in-hospital survival during time-cycled, volume preset AC with constant inspiratory flow (12). The study by Papazian et al. (12), however, was criticized for being underpowered (13) and did not provide a physiological mechanism for improved survival with NMB. Patients in both groups were deeply sedated and breathing pattern and lung mechanics [provided in Supplementary Appendix, page 11 (12)] at days 1, 2, and 7 did not differ appreciably. It is conceivable that patient-ventilator asynchrony might have occurred outside the data acquisition periods and that this may explain the higher rate of barotrauma and pneumothorax in the control group. Another possible mechanism includes a direct anti-inflammatory action of cis-atracurium by blocking the nicotinic acetylcholine receptor α1 of isolated human lung epithelial, endothelial, and CD14+ cells, that were challenged with mechanical stretch and in a rat model with acid aspiration lung injury (14).
On the other hand, even short term NMB treatment for 48 hours, as used in the study by Papazian et al. (12), may result in disuse atrophy of skeletal and respiratory muscles. In a landmark study in 14 brain dead organ donors, Levine and colleagues (15) demonstrated decreased cross-sectional areas of diaphragmatic slow-twitch and fast-twitch fibers by >50% following 18–69 hours of mechanical ventilation with total muscular inactivity. These ideas were consistent with increased diaphragmatic proteolysis during muscle inactivity noted by other authors and reproduced with extensions to include physiological and histobiochemical signs of diaphragmatic injury and atrophy (5). A prospective multicenter cohort study in 251 ventilated patients showed that early sedation depth independently predicted delayed extubation and increased mortality (16). Overall, a strategy to adapt the patient to the ventilator obviously has serious defects and does not take into account modern ventilator modes which have the ability to avoid patients “fighting the ventilator”.
Adapting the ventilator to the patient
In addition to previous data challenging the concept of deep sedation in ventilated patients (1,16,17), a study in patients with detected breath stacking asynchrony during volume-preset AC demonstrated that increasing the level of sedation was much less effective than adapting the ventilator to the patient by switching the mode to pressure support ventilation (18).
Modern ventilators are microprocessor controlled, equipped with demand flow valves, and offer a variety of different modes of augmentation for spontaneously breathing patients with respiratory failure. These include assisted pressure-controlled, volume controlled and pressure regulated settings, or switching between two levels of CPAP in a time-cycled manner (BIPAP/APRV). When using pressure regulated modes, VT should be monitored and controlled. Recent data, however, suggest that it is even more crucial to adjust and minimize driving pressure carefully (19,20), which can be further increased by synchronized negative pleura pressure fluctuations due to spontaneous breathing. Thus, strong diaphragmatic activity should be avoided (21) and the use of non-synchronized modes of augmentation (BIPAP, APRV) may be preferable (22). Non-synchronized modes proved to preserve VT variability and simultaneously provide effective prevention of potentially detrimental, very high VT (21).
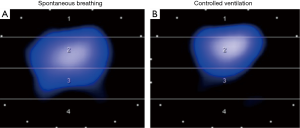
In contrast to AC, ventilator modes that offer high degree of freedom for interaction of a cooperative patient with the ventilator also allow sufficient augmentation of spontaneous breathing, precluding adequate analgesia and allowing for minimal sedation. This concept has several potential advantages: (I) the patient can be mobilized early, even in the acute phase of disease, and despite treatment of multiple organ failure (4,23,24); (II) since diaphragm muscle portions are located laterally and dorsally, spontaneous breathing activity redistributes regional ventilation to the dependent lungs (Figure 1) (25); (III) moderate spontaneous breathing activity may prevent ventilator induced diaphragm muscle atrophy and dysfunction (5,15); (IV) less sedation and spontaneous breathing activity may improve cardiorespiratory function (26).
In conclusion, we agree with Dr. Beitler and colleagues (9) that detecting and avoiding breath stacking is crucial. Instead of using early deep sedation and muscle relaxation, however, we suggest using ventilator modes that allow adaptation of the ventilator to the patient and not vice versa (27). This approach is compatible with the concept of early mobilization with no or early low-level sedation, which may decrease time in the intensive care unit, improve functional mobility at hospital discharge and may well improve outcome (28).
Acknowledgements
Many thanks to David Petroff, MSc, Clinical Trial Centre, University of Leipzig, Leipzig, Germany for his critical revision of the text.
Footnote
Conflicts of Interest: H Wrigge received research funding, lecture fees, and technical support from Dräger Medical, Lübeck, Germany; funding from Pfizer (Investigator Initiated Trial Program), Berlin, Germany; funding and lecture fees from InfectoPharm, Heppenheim, Germany; lecture fees from GE Healthcare, Freiburg, Germany, lecture fees from Maquet, Rastatt, Germany; lecture fees from MSD, Konstanz, Germany; and technical support from Swisstom Corp., Landquart, Switzerland. The other authors have no conflicts of interest to declare.
References
- Kress JP, Pohlman AS, O'Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000;342:1471-7. [Crossref] [PubMed]
- Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996;335:1864-9. [Crossref] [PubMed]
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371:126-34. [Crossref] [PubMed]
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874-82. [Crossref] [PubMed]
- Jaber S, Petrof BJ, Jung B, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 2011;183:364-71. [Crossref] [PubMed]
- Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 2013;188:220-30. [Crossref] [PubMed]
- Pohlman MC, McCallister KE, Schweickert WD, et al. Excessive tidal volume from breath stacking during lung-protective ventilation for acute lung injury. Crit Care Med 2008;36:3019-23. [Crossref] [PubMed]
- Gajic O, Frutos-Vivar F, Esteban A, et al. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med 2005;31:922-6. [Crossref] [PubMed]
- Beitler JR, Sands SA, Loring SH, et al. Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: the BREATHE criteria. Intensive Care Med 2016;42:1427-36. [Crossref] [PubMed]
- Thille AW, Rodriguez P, Cabello B, et al. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 2006;32:1515-22. [Crossref] [PubMed]
- Blanch L, Villagra A, Sales B, et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med 2015;41:633-41. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Horner D, Cairns C. Early neuromuscular blockade in severe ARDS. Journal of the Intensive Care Society 2011;12:153-4. [Crossref]
- Fanelli V, Morita Y, Cappello P, et al. Neuromuscular Blocking Agent Cisatracurium Attenuates Lung Injury by Inhibition of Nicotinic Acetylcholine Receptor-α1. Anesthesiology 2016;124:132-40. [Crossref] [PubMed]
- Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008;358:1327-35. [Crossref] [PubMed]
- Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med 2012;186:724-31. [Crossref] [PubMed]
- Mehta S, Burry L, Cook D, et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA 2012;308:1985-92. [Crossref] [PubMed]
- Chanques G, Kress JP, Pohlman A, et al. Impact of ventilator adjustment and sedation-analgesia practices on severe asynchrony in patients ventilated in assist-control mode. Crit Care Med 2013;41:2177-87. [Crossref] [PubMed]
- Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Yoshida T, Uchiyama A, Matsuura N, et al. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med 2012;40:1578-85. [Crossref] [PubMed]
- Richard JC, Lyazidi A, Akoumianaki E, et al. Potentially harmful effects of inspiratory synchronization during pressure preset ventilation. Intensive Care Med 2013;39:2003-10. [Crossref] [PubMed]
- Wrigge H, Zinserling J, Stüber F, et al. Effects of mechanical ventilation on release of cytokines into systemic circulation in patients with normal pulmonary function. Anesthesiology 2000;93:1413-7. [Crossref] [PubMed]
- Schaller SJ, Anstey M, Blobner M, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet 2016;388:1377-88. [Crossref] [PubMed]
- Froese AB, Bryan AC. Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 1974;41:242-55. [Crossref] [PubMed]
- Putensen C, Hering R, Muders T, et al. Assisted breathing is better in acute respiratory failure. Curr Opin Crit Care 2005;11:63-8. [Crossref] [PubMed]
- Wrigge H, Reske AW. Patient-ventilator asynchrony: adapt the ventilator, not the patient! Crit Care Med 2013;41:2240-1. [Crossref] [PubMed]
- Balzer F, Weiß B, Kumpf O, et al. Early deep sedation is associated with decreased in-hospital and two-year follow-up survival. Crit Care 2015;19:197. [Crossref] [PubMed]


