Does videomediastinoscopy with frozen sections improve mediastinal staging during video-assisted thoracic surgery pulmonary resections?
Introduction
Video-assisted thoracoscopic surgical (VATS) lobectomy, compared with lobectomy by thoracotomy, is associated with superior postoperative outcomes, fewer postoperative complications and better compliance with adjuvant chemotherapy (1-3). As a consequence, VATS lobectomy and major pulmonary resections during the last years have increased their popularity. Even if several and authoritative authors demonstrated the efficacy of VATS lobectomy in terms of oncological results and validity of mediastinal intraoperative staging, the issue is still debated (4,5). Particularly, the safety and effectiveness of VATS mediastinal lymphadenectomy outside specialty centres or during the learning curve period, is considered a critical point. An incomplete mediastinal intra-operative staging in non-small cell lung cancer (NSCLC), may result in an incorrect staging and patients would be denied significant chances of cure (i.e., adjuvant chemotherapy in stage IIA and higher).
Although no rigid standards exist for the conduct and extensiveness of mediastinal lymph node (LN) dissection, the topic of its improvement during VATS lobectomy or other major pulmonary resection (VMPR) is still controversial (6-10). A combined mediastinoscopic approach [video-assisted mediastinoscopic lymphadenectomy (VAMLA)] has been advocated to achieve a more satisfying radical minimally invasive lymphadenectomy during VATS lobectomy (11). However, VAMLA is not universally performed and it is a safe procedure only in experienced hands. Even if standard video-mediastinoscopy (VM) still remains the gold standard in current guidelines for pre-operative staging (12), its combination with VATS lobectomy, using frozen sections (FS) to decide whether to continue or to abort the procedure, to date has not been reported.
The present study was undertaken to evaluate if VM with FS combined with a same-day VATS lobectomy or other anatomic pulmonary resections is able to improve VATS intraoperative staging. As secondary endpoint, we also evaluated effectiveness of FS performed on VM samples.
Methods
Our institutional review board granted approval and waived the requirement for specific informed consent for this retrospective study. We selected patients scheduled for a VMPR for NSCLC with clinically negative N2 nodes in the period June 2012 to March 2015 at our institution (Thoracic Surgery Unit, University Hospital Careggi, Florence). Preoperatively, each patient was staged using thoracic computed tomography (CT) scanning and whole-body positron emission tomography (PET) scanning. The mediastinal LN were defined as being clinically negative if, on CT scan, they were ≤10 mm and were not hypermetabolic on PET scanning. Additional diagnostic tests were performed according to patient symptoms and clinical findings.
VM was indicated following the National Comprehensive Cancer Network (NCCN) guidelines (version 2.2010) (12), as it is routinely done at our institution for all NSCLC cases scheduled for a surgical treatment: T >3 cm, clinical N1 disease, central tumour. Endobronchial ultrasound (EBUS) was not available at our institution in that period.
VM always comprised sampling and/or dissection as much as possible of stations: 7, 2/4R, 2/4L. Once a station was sampled and dissected, a sheet fragment of oxidized regenerated cellulose (Tabotamp/Surgicel Original, Ethicon Inc. Somerville, NJ, USA) was left in the area of dissection, not only to improve hemostasis, but also to be a mark of the already dissected area and to make easier and faster the subsequent VATS lymphadenectomy. Stations dissected or sampled during VM have not been considered for VMPR lymphadenectomy results.
All VM specimens were sent from the operating theatre to the pathology laboratory for FS. LNs were macroscopically examined and frozen completely in Optimal Cooling Temperature (OCT) media in a cryostat machine at −20 to −25 °C. The frozen block was aligned and faced to avoid waist nodal tissue, and two to four (depending on the size of the LNs) 6 µm thick sections were cut with the microtome from the frozen block. Sections were immediately stained with hematoxylin and eosin (H&E) and interpreted by the pathologist. For permanent sectioning, the frozen block was thawed, fixed in neutral buffered formalin, and completely submitted for permanent sectioning. After paraffin embedding, 4 µm sections were prepared and stained with H&E.
In the meantime, the patient was prepared in the lateral decubitus for the subsequent VATS procedure; resection was carried out only if the FS results were negative in all samples.
The VATS procedure was performed via a 3-cm utility thoracotomy in the fourth and two port incisions along the seventh or eighth interspace. The thoracoscopic lymphadenectomy followed the NCCN (version 2.2010) recommendations (12): minimum of 3 N2 stations sampled or complete LN dissection. All VATS mediastinal lymphadenectomy specimens underwent routine histopathological and immunohistochemical examination. Number, location and definitive histological assessment of the dissected N2 and N1 nodal stations were evaluated. Nodal upstaging was reported as the number and percentage of patients who were found to have LN metastasis in the surgical specimen after being clinically understaged (cN0 to N1/N2; cN1 to N2), based on preoperative CT and PET scans and upon mediastinoscopy in those patients who underwent VM.
Operation time was measured from incision to suture. In the VM + VATS group, we considered the procedure time between VM skin incision and VMPR skin closure.
VATS conversions and adverse events were documented, as well as complications VM related.
Statistical analysis was performed using SPSS 16.0 software (SPSS, Chicago, IL). Continuous variables are expressed as mean values ± SD or median and range. Categorical variables were analysed using Fisher’s exact test or χ-square test. Continuous variables were compared by Student’s t-test or Mann-Whitney test. A P value <0.05 was considered statistically significant.
Results
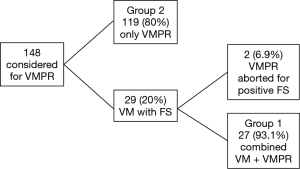
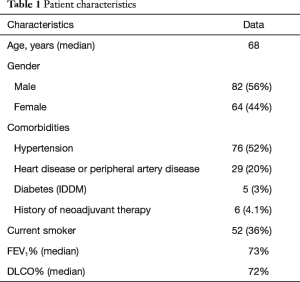
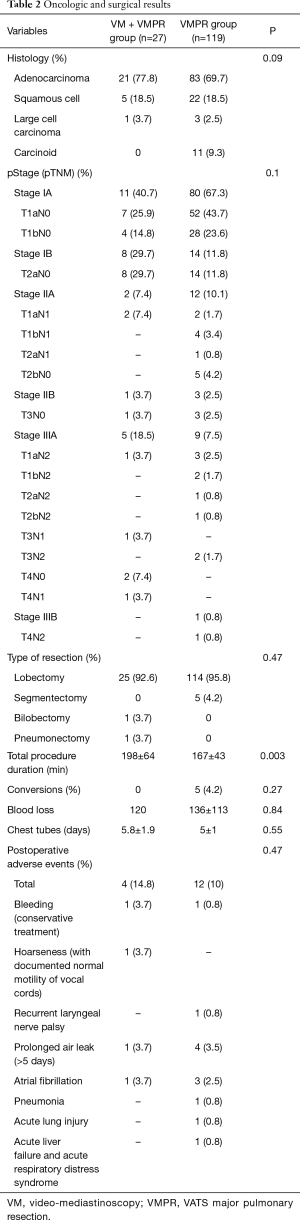
Figure 1 shows the schema of results of our VMPR program. Patient characteristics are depicted in Table 1. A total of 146 patients underwent VATS anatomical lung resection for lung carcinoma; they were: lobectomy 139 (95.2%); bilobectomy 1 (0.7%); left pneumonectomy 1 (0.7%); segmentectomy 5 (3.4%). Group 1 (VM + VMPR) consisted of 27 patients (18.5%), group 2 (VMPR alone) consisted of 119 patients (81.5%). Detailed oncologic and surgical results are given in Table 2.
Full table
Full table
Both groups were quite balanced for histology, even if we found carcinoid tumours only in group 2. Since group 1 was selected on the basis of the need for an invasive mediastinal staging using the NCCN guidelines (more advanced clinical stages) (12), differences (even if not statistically significant) were observed on pTNM distribution and type of resection. Particularly, in group 1 we performed: 1 (3.7%) superior bilobectomy, for a double lesion of the upper and middle lobe, clinically N0; 1 (3.7%) left pneumonectomy again for a double lesion involving respectively the upper and lower lobe, in both cases until the origin of the lobar bronchus. In group 2, we performed only lobectomies ad segmentectomies. We observed a total of 4 (2.7%) pT4 lesions: 3 (2%) belonging to the category “separate tumour nodule in a different ipsilateral lobe”; 1 (0.7%) belonging to the category “tumour invading the mediastinal fat”, in a patient operated after a good response to induction chemotherapy. About the clinical parameters (operation time, intraoperative and postoperative adverse events, conversions, blood loss, drainage time) (Table 2), we found a significant difference between the VMPRS and VM + VMPR group only in operation time. No 30-day post-operative mortality was observed in both groups. No complications related to VM were observed in group 1, if we exclude a case of postoperative hoarseness, initially interpreted as recurrent laryngeal nerve palsy, but subsequently excluded by a documented normal vocal cord motility. One case (0.8%) of recurrent laryngeal nerve palsy occurred in group 2, in a left-sided procedure, probably linked to station 5/6 dissection.
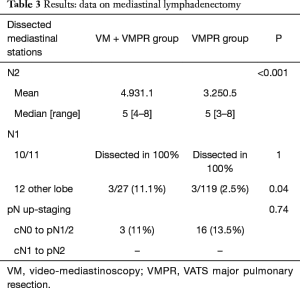
With regard to mediastinal lymphadenectomy (Table 3), the mean and the median (range) number of dissected mediastinal LN N2 stations resulted as significantly higher in group 1. No differences were observed about N1 stations, since LN dissection achieved rates of 100% for both, stations 10 and 11.
Full table
To assess the range of group 1 vs. group 2 N2 LN dissection, the yield of either methods was expressed as dissection proportion (percentage of patients with dissected station) per station, and stratified for right- and left-sided resections (Figures 2,3). Station 8 and 9, station 2R and 4R, station 3a and 3p in right-side procedures and station 5 and 6 and 8 and 9 in left-sided procedures, were usually dissected en-bloc or however removed contemporarily, so that they have been considered together. The combined VM + VMPR approach improved dissection proportions for any mediastinal station.
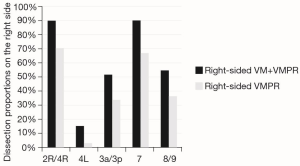
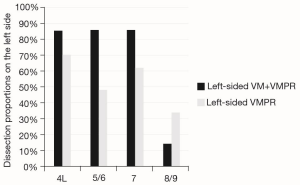
Pathological upstaging (pN1; pN2) is depicted in Table 3; in 1 (3.7%) patient of group 1, upstaging (cN1 to pN2) was due to a positive paraesophageal station, not sampled during VM. Considering the two cases of aborted lobectomy for positive FS, upstaging in VM patients increases to 5 (18.5%). About FS performed during VM (n=29), no false-negative or false-positive were observed.
Discussion
In our study, we compared the completeness of the LN evaluation between VMPRs combined with standard VM and VMPR alone in patients with NSCLC. Secondarily we evaluated if FS of VM samples are as effective as formal paraffin assessment for a decision on a same-day VMPR.
In the last few years, VATS major pulmonary resections have gained popularity worldwide. A wide number of reports have demonstrated that VATS lobectomy can be performed with minimal complications and a shorter length of stay compared with open lobectomy (1-5). However, several thoracoscopic approaches and technical options have been described: the number of ports (uniportal vs. bi- or three-port technique), the size and position of the incisions, the different approach to the hilum (anterior, posterior, inferior) and the amount of anatomical hilar and interlobar dissection are main topics of an ongoing discussion or a matter of choice for the surgeon approaching VATS lobectomy (13-16). We evaluated VATS lymphadenectomy during VMPR on a series of 146 consecutive patients, following the same pre-operative indications for invasive mediastinal staging (NCCN 2010 guidelines), and operated with the same standard three-port anterior approach (15): a <5 cm utility incision, without rib spreading or soft tissue retraction; other two ports (<1.5 cm), placed one anteriorly at the level of the diaphragm and one posteriorly at the same level in a straight line down from the scapula.
Despite benefits of VATS lobectomy, the completeness of LN evaluation during the procedure has been questioned. From the oncological point of view, the main topic is whether and to what extent a LN dissection and an effective intraoperative staging is feasible, as minor mediastinal involvement is possible in more than 15% of clinical stage IA and thus VATS resectable NSCLC (17). Currently, the ideal extent of LN dissection during VATS lobectomy for lung carcinoma remains unknown and controversial. In our series, we uniformly followed the 2010 NCCN guidelines (12), which suggests a minimum of 3 N2 stations to be sampled or dissected.
Several authors compared lymphadenectomy during VATS and open surgery and found similar results in terms of number of LNs, number of sampled or dissected stations and overall survival. D’Amico, et al. (17) analyzed the NCCN NSCLC database to compare the efficacy of mediastinal LN dissection during lobectomy by VATS (199 patients) and open thoracotomy (189 patients) and at least three mediastinal LN stations were assessed in most patients who underwent lobectomy by either approach; as evaluated by the number of LN stations, there was no difference in the efficacy of LN dissection by approach.
Watanabe et al. (13) evaluated the effectiveness of VATS LN dissection in terms of number of dissected LNs per station, comparing a VATS lobectomy group of 221 patients with an open thoracotomy group of 190 patients; no significant differences were observed with regard to number of dissected nodes between the VATS group and open thoracotomy group.
If it is true that many studies focused on comparing VATS vs. open thoracotomy lymphadenectomy, it is equally true that inside the group of VATS lymphadenectomy, few papers analyzed technical elements or other perioperative strategies that can be able to affect effectiveness of VATS LN dissection and intraoperative mediastinal staging. As an exception, Witte et al. (11) evaluated the feasibility and radicality of a combined thoracoscopic and mediastinoscopic approach to improve minimally invasive mediastinal LN dissection: they compared the number of dissected stations and sample weight in a group of 14 patients who underwent VATS lobectomy alone versus a group of 18 patients who underwent a combined VAMLA and VATS lobectomy. They concluded that VAMLA improves radicality of minimally invasive mediastinal lymphadenectomy without increasing operation time, morbidity, and drainage time. However, VAMLA is not so popular and easily reproducible. On the contrary, standard VM is universally known and routinely performed; therefore, we decided to evaluate if the combination of a standard VM and VATS lobectomy could be able to improve mediastinal lymphadenectomy.
Our assumption was that dissection performed during VM could improve lymphadenectomy during the immediately following VATS procedure for several reasons:
- The anatomical space is already prepared and can be marked with Tabotamp or similar products for the subsequent VATS;
- LN dissection can be partially performed during VM and just completed during VATS, particularly for station 7 on the left;
- Particularly during the learning curve, lymphadenectomy can be felt as tedious, time-consuming and it comes at the end of lobectomy, finding the surgeon quite tired an stressed. VATS dissection of paratracheal and subcarinal stations is easier and faster after VM, so that the surgeon can dedicate more time and energies during VATS to dissect other stations not involved by VM dissection. For this reason, we found an improved lymphadenectomy also in stations not involved by the previous VM, such as stations 3a/3p, stations 5/6 and stations 8/9.
We decided not to consider samples removed during VM in the final result of the group VM + VMPRS:
- Because sampling performed during VM is not dissection as it is performed during VATS and in our study we want to focus on intraoperative VATS lymphadenectomy (dissection);
- Because we want to evaluate if pre-operative VM facilitates VATS lymphadenectomy, in terms of number of dissected stations during thoracoscopic pulmonary resection: considering stations sampled during VM (subcarinal and paratracheal stations bilaterally) together with the stations dissected during intra-operative lymphadenectomy it would not be correct and would invalidate the results.
Witte in her study (11) observed that the combined VATS + VAMLA approach improved dissection proportions for any mediastinal station except station 9. For left-sided procedures, this effect was more pronounced. For the stations 3, 4R + L, and 7, VATS + VAMLA achieved dissection proportions of 100%.
We observed the same increase in effectiveness of LN dissection for every mediastinal station, without observing an increase of VM related complications; in group 1, if we had included data of samples removed during VM, we also would achieve sample rates of 100% for paratracheal and subcarinal stations.
With regard to total number of N2 stations (Table 3), the mean number of dissected mediastinal N2 LN stations was significantly higher in the VM + VMPR group. Also, the median of dissected stations and its range resulted as statistically significantly higher in group 1.
About N1 stations (Table 3), VATS hilar dissection has brought to an accurate isolation and hilar lymphadenectomy. In this series, station 10 and 11 were always dissected. About station 12, we’ve only considered and mentioned the cases of dissected peribronchial stations belonging to a different lobe.
From the logistic point of view, Witte et al. (11) suggested a staged procedure, with VAMLA performed first, only if an accurate mediastinal staging was important (adenocarcinoma, T >2 cm); otherwise they chose for a timing suitable for the individual clinical context. We instead opted for a same-day sequence: VM with FS of LN samples, immediately followed by VMPR if FS were negative. This kind of combined procedure was first proposed by Gephardt and Rice in 1990 (18); they reported the results of FS with histology from 122 consecutive patients with NSCLC and showed no false-positive results and only 1.6% false-negative in the samples. They also showed a 15% lower cost with such single-stage procedure. However, the use of VM and FS has not gained popularity, and Attaran et al. (19) in a recent review [2013], found only five papers comparing results of mediastinal FS with formal paraffin embedded histopathology results (18-21); based on this review, they conclude that:
- FS of mediastinal LNs is as sensitive and specific as definitive histological evaluation and therefore, a combined procedure of mediastinal LN biopsy ± lung resection should be considered;
- This approach is preferred by the patients due to single hospitalization and single anaesthesia;
- The rate of false-positive with FS was 0% in all the studies so that the risk of abandoning lung resection in a resectable lung cancer is expected to be zero;/
- Only a small percentage of FS were false-negative and these cases may be due to micro-metastasis and limited mediastinal LN involvement in which lung resection remains beneficial. In our study, we experienced the same: the formal paraffin assessment always confirmed the FS result. Regarding the economic aspect, we assume that additional material costs of the combined surgery are balanced by concentrating the two procedures in a same day-surgery, with the same equip performing both, VM and the subsequent VMPR; as expected operating theatre occupation time increases significantly.
To further analyze the efficacy of mediastinal lymphadenectomy, several authors evaluated the degree of upstaging during VATS lobectomy and they found rates between 5.3% and 16.6% (6-8,10,17,22,23): our 13.5% in group 2 is in line with these data; the 11% of group 1, that remains an high rate despite the previous VM, may be due not only to an accurate lymphadenectomy, but also to the selection criterion of these patients [more at risk from an unforeseen N2 disease, following the NCCN indications (12)].
We experienced 14.8% and 10% of 30-day complication rate in group 1 and group 2 respectively which is in line with other series (1-4,13,17,24). In group 1, 1 (3.7%) patient referred a post-operative hoarseness, that we linked to VM, even if vocal cords were apparently normal; the symptom resolved spontaneously in few weeks. Our study has several limitations:
- We compare a group of 27 patients (group 1, VM + VMPRS) with a group of 119 patients (group 2, VMPR alone);
- Patients belonging to group 1 have been selected on the basis of the 2010 NCCN guidelines for pre-operative mediastinal staging; it means that they are patients where an accurate mediastinal staging is crucial. In this setting, the surgeon may be brought to perform a more accurate VATS lymphadenectomy, regardless of previous VM;
- Unfortunately our pathology service does not provide the number or the weight of LNs removed per station. The analysis of these data may include additional information.
Based on our experience, we conclude that, even with all the limits of the present study, the combination “VM with FS followed by VMPRS in a same-day surgery sequence”, seems to be safe and effective, and it offers an efficient approach to improve intraoperative mediastinal staging.
Acknowledgements
None.
Footnote
Conflicts of Interest: Presented at the 29th European Association for Cardio-Thoracic Surgery (EACTS) Annual Meeting, 3–7 October 2015, Amsterdam. Session: T3081 - Thoracic oncology I: Staging (Monday, October 5, 2015 Time: 8:15 AM–9:45 AM).
Ethical Statement: Our institutional review board granted approval and waived the requirement for specific informed consent for this retrospective study.
References
- Laursen LØ, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg 2016;49:870-5. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 1250. [Crossref] [PubMed]
- Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014;46:607-13. [Crossref] [PubMed]
- Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for N0 lung cancer. Ann Thorac Surg 2013;96:1171-7. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [Crossref] [PubMed]
- Amer K, Khan AZ, Singh N, et al. Video-assisted thoracic surgery systematic mediastinal nodal dissection and stage migration: impact on clinical pathway. Eur J Cardiothorac Surg 2011;40:1474-81. [PubMed]
- Nosotti M, Cioffi U, De Simone M, et al. Systematic mediastinal nodal dissection and video-assisted thoracic surgery. Eur J Cardiothorac Surg 2012;42:385-6; author reply 386-7. [Crossref] [PubMed]
- Reichert M, Steiner D, Kerber S, et al. A standardized technique of systematic mediastinal lymph node dissection by video-assisted thoracoscopic surgery (VATS) leads to a high rate of nodal upstaging in early-stage non-small cell lung cancer. Surg Endosc 2016;30:1119-25. [Crossref] [PubMed]
- Witte B, Messerschmidt A, Hillebrand H, et al. Combined videothoracoscopic and videomediastinoscopic approach improves radicality of minimally invasive mediastinal lymphadenectomy for early stage lung carcinoma. Eur J Cardiothorac Surg 2009;35:343-7. [Crossref] [PubMed]
- Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2010;8:740-801. [PubMed]
- Watanabe A, Koyanagi T, Ohsawa H, et al. Systematic node dissection by VATS is not inferior to that through an open thoracotomy: a comparative clinicopathologic retrospective study. Surgery 2005;138:510-7. [Crossref] [PubMed]
- Pompeo E. State of the art and perspectives in non-intubated thoracic surgery. Ann Transl Med 2014;2:106. [PubMed]
- Hansen HJ, Petersen RH, Christensen M. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc 2011;25:1263-9. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- D'Amico TA, Niland J, Mamet R, et al. Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann Thorac Surg 2011;92:226-31; discussion 231-2. [Crossref] [PubMed]
- Gephardt GN, Rice TW. Utility of frozen-section evaluation of lymph nodes in the staging of bronchogenic carcinoma at mediastinoscopy and thoracotomy. J Thorac Cardiovasc Surg 1990;100:853-9. [PubMed]
- Attaran S, Jakaj G, Acharya M, et al. Are frozen sections of mediastinoscopy samples as effective as formal paraffin assessment of mediastinoscopy samples for a decision on a combined mediastinoscopy plus lobectomy? Interact Cardiovasc Thorac Surg 2013;16:872-4. [Crossref] [PubMed]
- de Montpréville VT, Dulmet EM, Nashashibi N. Frozen section diagnosis and surgical biopsy of lymph nodes, tumors and pseudotumors of the mediastinum. Eur J Cardiothorac Surg 1998;13:190-5. [Crossref] [PubMed]
- Sanli M, Isik AF, Tuncozgur B, et al. The reliability of mediastinoscopic frozen sections in deciding on oncological surgery in bronchogenic carcinoma. Adv Ther 2008;25:488-95. [Crossref] [PubMed]
- Daniels LJ, Balderson SS, Onaitis MW, et al. Thoracoscopic lobectomy: a safe and effective strategy for patients with stage I lung cancer. Ann Thorac Surg 2002;74:860-4. [Crossref] [PubMed]
- Shapiro M, Kadakia S, Lim J, et al. Lobe-specific mediastinal nodal dissection is sufficient during lobectomy by video-assisted thoracic surgery or thoracotomy for early-stage lung cancer. Chest 2013;144:1615-21. [Crossref] [PubMed]
- Rueth NM, Andrade RS. Is VATS lobectomy better: perioperatively, biologically and oncologically? Ann Thorac Surg 2010;89:S2107-11. [Crossref] [PubMed]


