Utility of thrombophilia testing in patients with venous thrombo-embolism
Introduction
Thrombophilia is an acquired or congenital abnormality of haemostasis predisposing to thrombosis. A thrombophilia screen panel in Queensland public hospitals includes protein C and protein S levels, antithrombin III level, lupus anticoagulant, activated protein C resistance (factor 5 Leiden mutation tested if positive or if specifically requested) and prothrombin gene mutation (PG202A). Antiphospholipid antibodies (anti-cardiolipin antibody and beta 2 glycoprotein antibody) require specific requests.
Thrombophilias are associated with an increased risk of VTEs. It is estimated that up to 4% of the population can have thrombophilia (1). Not all thrombophilias are equal in terms of their relative risks for thrombotic events. Middeldorp et al. (2) has summarised data from multiple studies which evaluated risks of first and recurrent episodes of VTEs from various thrombophilic conditions which showed most conditions to have relative risks from 1 to 10 for first event, and from 1 to 2.6 for recurrent event. Notably, the risk of recurrent VTEs is particularly high for antiphospholipid antibodies, showing up to 6 times the relative risk (2), making long-term anticoagulation with vitamin K agonist (warfarin) a mainstay of management in these patients (3).
International guidelines (4-7) have recommendations on thrombophilia testing on patients with VTEs. It is not clear whether the current practice in public hospitals is concordant with these guidelines. For instance, it is not recommended by guidelines (4-7) to perform the thrombophilia screen in the setting of venous thrombosis in patients with clearly established risk factors such as recent surgery or active malignancy. The most recent guideline (6) also suggests that thrombophilia screen not be performed on patients with unprovoked VTEs, as results of this screen do not alter the management strategy. This is reflected on the American College of Chest Physicians (ACCP) guideline (7), where it is recommended to treat patients with unprovoked VTEs with long-term anticoagulation. This is further confirmed in the updated guideline (8). In the 2012 guideline (7), it is stated that thrombophilia conditions predict risk of recurrence, but not strongly or consistently enough to influence recommendations on duration of therapy. European Society of Cardiology (ESC) guideline (9) also follows this recommendation. In summary, guidelines (4-7) are mostly recommending against the use of thrombophilia screen to predict the risk of further VTEs in patients who present with an episode of acute VTEs. Thrombophilia screen should only be ordered in a highly selected patient group, such as in patients with strong family history of recurrent unprovoked VTEs (4), although even in these patient groups there is no clear recommendation to perform thrombophilia screen (4). Thrombophilia screen is a highly specialised test which requires careful consideration of the individual patient’s clinical history, treatment choices and preferences, and should not be used in unselected group of patients who present with an episode of acute VTEs.
We hypothesized that clinical practice of thrombophilia ordering is inconsistent with these guidelines, with widespread testing being performed on unselected patients who present with acute VTEs. While the test is frequently ordered and performed, it is not clear whether these results have actually contributed to clinical decision making. Specifically, we wished to address the question whether investigating for thrombophilias for patients with VTEs does results in a change to the patient’s management.
The purpose of this study is to review the data of patients presenting with VTEs to two public hospitals to analyses the utility of thrombophilia screen. In detail, the patient characteristics, rate of thrombophilia testing, yield of tests in different population groups and proportion of results that lead to change in management of the patients were analysed. Our hypotheses were that: (I) practices of clinicians are not consistent with the guidelines; (II) thrombophilia testing are not utilised to lead clinical decision making.
Methods
This multi-centre retrospective study was performed via chart review of patients’ records from two public hospitals in Queensland. The Prince Charles Hospital (TPCH), the tertiary cardiothoracic and general hospital in Brisbane, and Mackay Base Hospital (MBH), a regional level 1 centre in North Queensland. Data were collected by one investigator reviewing the medical records of patients who presented to both hospitals with VTEs from September 2011 to August 2012 (including patients who were either admitted or discharged home from the ED).
Institutional ethics committee approvals were obtained. No patient consent was sought for this study. The health information management division of each hospital provided a list of all patients who presented to the hospital acutely with venous thromboembolism, using standardized searching of diagnostic codes—“Embolism & thrombosis other specified veins”, “Phlebitis & thrombophlebitis femoral vein”, “Pulmonary embolism without acute cor pulmonale”, “Phlebitis & thrombophlebitis other deep vessel legs”. These include patients who presented to the emergency department (ED) and then were discharged, admitted, or those who were admitted directly without ED involvement. Patients who were purely treated as an outpatient in clinic setting were not included in this study. These lists were then forwarded to medical records to provide charts for the review. All the 69 cases identified in MBH were analyzed and included in this study, as it is a smaller regional hospital compared with TPCH, a large tertiary referral hospital. Out of the 502 cases identified at TPCH during the study timeframe, 83 were randomly selected by the randomization function of Microsoft Excel for analysis. This number for TPCH was selected to provide an approximately similar sample size of representative patients, compared to the MBH cohort. During the data collection, information from charts, electronic discharge summary, electronic pathology results and imaging results were utilised.
The following data were collected for analysis: patient demographics, risk factors, diagnoses (classified as DVT, PE or both confirmed on imaging), previous history of VTEs, previous thrombophilia screen results, thrombophilia results, duration of treatment as intended by treating team, follow-up strategy, and whether duration of anticoagulation was altered as a result of thrombophilia testing. Patient details were coded and stored in secured electronic location protected by a password.
The results of thrombophilia were analysed as either positive or negative for the presence of hereditary genetic mutations. In the cases of natural anticoagulant deficiencies (protein S, C and anti-thrombin III), one test result with the low level is not enough for diagnosis of true deficiency status (4), as proteins S and C levels are affected by warfarin therapy (10,11), and all natural anticoagulant levels are affected by acute thrombosis (12,13). Therefore, natural anticoagulant deficiency was only considered to be confirmed if more than one test was performed with a second test definitively showing persistently low levels at least after 4–6 weeks post completion of the anticoagulant therapy. However, due to the retrospective nature of this study, and the limited window of the study period, all cases for low levels of natural anticoagulants were recorded for analysis of the study, even if there was only one result available at the time of data collection. For antiphospholipid syndrome, international consensus (Sydney) classification (ICS) criteria (14) for definite antiphospholipid syndrome were utilised.
Statistical analysis of the rate of positive thrombophilia tests between the provoked vs. the unprovoked group and the first event vs. the recurrent event groups was performed using Chi-square tests. A P value of <0.05 (two-tailed) was considered statistically significant.
Results
A total of 152 patients presented with VTEs in the defined one year study period were analysed, with 69 from MBH and 83 from TPCH. The patients’ characteristics are presented in Table 1. Mean age was 58 (range, 15–87 years) with standard deviation of 18. There were more males (57%) than females in this study. Pulmonary embolism alone was the most common diagnosis with 61%, followed by DVT alone with 31%, with combined Dx of DVT and PE at 9%.
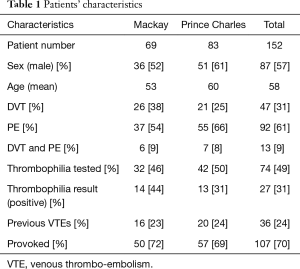
Full table
Overall, 74 patients (49%) were tested with thrombophilia screen with 27 patients returning positive results (37%). All the tests were done during the admission and all were done within 7 days of diagnoses. For factor V Leiden mutation testing, this was only specifically asked in five cases and none identified a new case of mutation. Figure 1 demonstrates the results of positive tests, with heterozygous factor V Leiden being the most common result with ten patients being tested positive. Three patients had more than one positive result. One patient had a compound heterozygous prothrombin and factor V Leiden mutation, one patient had antiphospholipid antibodies as well as heterozygous factor V Leiden mutation, and one patient had a low protein C level and a heterozygous factor V Leiden mutation. All 3 patients were placed on long-term anticoagulation with specialist follow-up arranged. In the case of positive APLa and F5L, this patient was 1 of the 2 cases identified where result of the thrombophilia screen changed duration of anticoagulation. In other 2 cases, the decision for long-term anticoagulation was made prior to the results became available because of either unprovoked episode of major VTE or because of recurrent unprovoked VTE. For the 11 patients returning low levels of protein S, C and anti-thrombin III levels, none fulfilled diagnostic criteria for these natural anticoagulant deficiencies, as no follow-up studies were completed during the period of data collection.
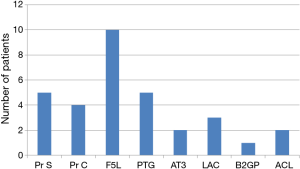
Of the 74 patients who were tested for thrombophilia, 23 patients (31%) were additionally tested for anti-phospholipid antibodies. However, it is noted that in 11 of these cases (48%) beta-2 glycoprotein was not ordered (i.e., only anti-cardiolipin antibody was tested). Two patients were known to have antiphospholipid syndrome with recurrent episode of VTEs with 1 of those patients already on permanent warfarin therapy. Two patients had a new positive lupus anticoagulant and were subsequently diagnosed with antiphospholipid syndrome on long-term anticoagulation. These 2 patients were in provoked group as both had periods of immobility due to hospital admission recently to the diagnosis. The testing for thrombophilia was done on one of these patient as he was young (25 years) and on the other patient as a known case of systemic lupus erythematosus (SLE) as there is an association between SLE and APLS (15-18).
There were two cases of documented change of duration of anticoagulation from the results of thrombophilia screen. In both cases, duration of anticoagulation was changed to long-term from initially planned 6 months. There was no identified case where negative thrombophilia result lead to a changed duration of anticoagulation. Proposed durations of anticoagulation as documented in charts are summarised on Table 2. The most common case was undetermined or not documented on the charts at 38%, followed by planned for 6 months with 30% and indefinite duration with 18%. Out of those patients placed on indefinite anticoagulation, 5 out of 12 (42%) patients who were tested returned positive thrombophilia result.
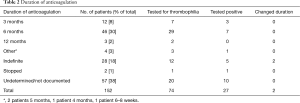
Full table
One hundred and seven patients were identified to have risk factors which have contributed to the development of VTEs and were classified as provoked event. Out of these patients, 41 were tested for thrombophilia and 12 (29%) tested positive. Forty-five patients, in contrast, presented with no apparent risk factors at the time of presentation and were therefore classified as unprovoked event. Out of these patients, 33 were tested for thrombophilia and 15 (45%) tested positive. The proportion of patients testing positive for thrombophilia between 2 populations showed a non-statistically significant trend to a higher rate in the unprovoked group compared to the provoked group (P=0.054), as presented on Table 3.
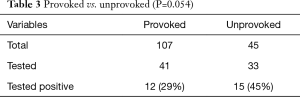
Full table
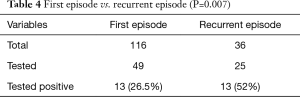
One hundred and sixteen patients presented with the first episode of VTEs while 36 patients presented with recurrent events of VTEs. Forty-nine out of 116 patients with the first episode of VTEs were tested for thrombophilia of which 13 (26.5%) returned positive results. In contrast, 25 out of 36 patients with recurrent VTEs were tested and 13 (52%) returned positive results. There was a statistically significant difference between the rates of positive thrombophilia results between these two groups with a higher rate of positive results seen in the recurrent group (P=0.007), as presented on Table 4.
Full table
Discussion
The current study analysed the practice of thrombophilia screening in patients who presented to two public hospitals in Queensland with the VTE. According to international guidelines (4-7) it is not recommended to perform screening in patients who had provoked episodes of VTEs. Overall, the rate of patients who received screening in this setting was 38% (41/107), with similar rates between two hospitals. This means that a significant proportion of patients were tested despite the guidelines to not test. Given there was no evidence of altering anticoagulation duration, it appeared that no significant clinical benefits were obtained as a result of screening, thus vindicating the guidelines. Also, 9 patients (8% of recurrent patients) in total were tested more than once (all on two separate admissions) with 5 cases being negative twice, with 2 cases of positive F5L, 1 case of low pr S level, and 1 case of low AT3 level. In cases of low pr S and AT3 levels, repeated tests were done but not within the appropriate timing, i.e., either during acute thrombosis or while patient on warfarin. In all cases, previous results, which were available on electronic pathology records, were seemingly not reviewed prior to these tests being ordered the 2nd time.
The group analysis of provoked vs. unprovoked groups showed a non-statistically significant trend (P=0.054) of a higher rate of positive results in the unprovoked group. It is likely that the main reason for this difference not reaching statistical significance is a type 2 error.
The clinical significance of the above results is uncertain in terms of whether a thrombophilia screen should be performed on patients with unprovoked VTEs. The prospective ELATE trial showed that the probability of recurrence in those with unprovoked VTEs with one or more inherited thrombophilic defects was the same as, or lower than, the recurrence rate in those with no abnormality during the entire length of the study from 3 months through more than 3 years (19). This indicates that there is no evidence to suggest that patients with VTEs with positive thrombophilia should be placed on long-term anticoagulation for that reason alone. This is further supported by other trials (20-22), which showed that while thrombophilias do seem to show increased risk of recurrence of VTEs in unprovoked VTEs, the fact those patients had unprovoked VTE itself conferred a greater risk of recurrence. These findings have now lead to guidelines suggesting against routinely testing for thrombophilias in patients with unprovoked VTEs (6,7). As ELATE trial did show an increased trend of recurrence of VTEs in setting of APLS (19), it is currently uncertain as to whether APLa studies should be done on patients who initially present with unprovoked VTEs, as it is possible that they should be placed on long-term anticoagulation. Further follow-up prospective studies need to be done to evaluate this question further.
In the analysis of recurrent vs. first episode of VTEs in this study, there was a statistically significant difference favoring the recurrent group (P=0.007). In these cases, long-term anticoagulation is required for those with low to moderate risk of bleeding (7,8). While the guidelines (4-9) make no specific recommendation regarding whether this group of population needs testing for thrombophilia, according to the result of ELATE, it is possible to argue that testing for thrombophilias in patients with recurrent episodes of VTEs are not warranted (19). The argument follows that if hereditary thrombophilia results do not change the recurrence rate of VTEs, then the decision for duration of anticoagulation should be made regardless of its result. This is consistent with the finding of this study which showed that in 82% (23 out of 28) of patients who were placed on indefinite anticoagulation, this decision was made with either a negative result or without being tested for thrombophilias. Again, the question of whether acquired thrombophilia should be tested in these patients requires further evaluation.
Overall, only 1.3% (2/152) of patients had documented evidence of a changed duration of anticoagulation due to the results of thrombophilia screen. Both patients were tested positive for APLa and were placed on long-term anticoagulation after diagnoses of APLS were established. In the other two APLS patients, the duration of anticoagulation intended were not specified in the charts. While positive results did not seem to change management, it is possible that negative results may have influenced physicians to not pursue long-term anticoagulation. However, documentation was missing in these cases, meaning that there was no evidence found to suggest that negative thrombophilia results influenced physicians’ decision making with regards to the duration of anticoagulation. Importantly, an anticoagulation plan was not documented in 38% of cases. This was a major gap in clinical practice that we observed, as documentation of the intended duration of anticoagulation is reasonably expected as a standard practice for management of VTEs. This also meant that in these patients, it was not possible to assess whether the results of thrombophilia screen have influenced duration of anticoagulation, creating a gap in our data. Ultimately, in some cases, negative thrombophilia results may have shortened the planned duration of anticoagulation, but there was no documentation found to state this practice.
Limitations of this study relate to the relatively small sample size. This is likely the main reason for provoked vs. unprovoked group analysis not reaching statistical significance. The 83 cases out of 503 identified at TPCH were therefore a subgroup of the whole TPCH cohort. However, as randomisation was performed to select the cases, it is reasonable to say that overall, it accurately reflects the contemporary practice of thrombophilia testing in this hospital. Further follow-up studies in these hospitals, including the follow-up audit and the intervention study attempting to reduce overutilisation of thrombophilia testing, are needed. Another limitation is the retrospective study design. Also, it is important to note that while they were included in the analysis, none of the cases of low natural anticoagulant levels were diagnostic for true natural anticoagulant deficiency disorder as the follow-up tests were not performed to confirm diagnoses. It should also be noted that there is difficulty in creating generalised recommendations on thrombophilia testing which includes multiple conditions, including hereditary and acquired thrombophilias. This is further complicated by the multi-factorial nature of the VTE itself, with a different clinical significance attached to PEs and DVTs.
In conclusion, clinical practice in public hospitals does not always reflect the guidelines, in particular with a significant proportion of patients with provoked VTEs being tested (38%). This suggests excessive testing is being conducted in general. These points may be addressed by creation and distribution of clinical practice guidelines, by conducting education sessions or by the means of clinical decision making tool. In the study published in 2014 (23), introduction of pre-printed order form outlining limitations of the study was required to be filled out by physicians prior to ordering thrombophilia, which lead to a significant decline in the rate of thrombophilia ordering. Similar intervention trials need to be performed in these institutions to improve clinical practice and address overutilisation of thrombophilia testing.
Acknowledgements
The authors would like to thank Suzanne Kuys (Schools of Allied Health Sciences, Griffith University) for statistical analysis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Protocol for the research project has been approved by a suitable Ethics Committee of the institutions within which the work was undertaken.
References
- MacCallum P, Bowles L, Keeling D. Diagnosis and management of heritable thrombophilias. BMJ 2014;349:g4387. [Crossref] [PubMed]
- Middeldorp S, van Hylckama Vlieg A. Does thrombophilia testing help in the clinical management of patients? Br J Haematol 2008;143:321-35. [Crossref] [PubMed]
- Erkan D, Aguiar CL, Andrade D, et al. 14th International Congress on Antiphospholipid Antibodies: task force report on antiphospholipid syndrome treatment trends. Autoimmun Rev 2014;13:685-96. [Crossref] [PubMed]
- Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010;149:209-20. [Crossref] [PubMed]
- Howard LS, Hughes RJ. NICE guideline: management of venous thromboembolic diseases and role of thrombophilia testing. Thorax 2013;68:391-3. [Crossref] [PubMed]
- Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis 2016;41:154-64. [Crossref] [PubMed]
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e419S-94S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315-52. [Crossref] [PubMed]
- Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033-69, 3069a-3069k.
- Hirsh J, Fuster V, Ansell J, et al. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation 2003;107:1692-711. [Crossref] [PubMed]
- Fair DS, Marlar RA. Biosynthesis and secretion of factor VII, protein C, protein S, and the Protein C inhibitor from a human hepatoma cell line. Blood 1986;67:64-70. [PubMed]
- Heit JA. Thrombophilia: common questions on laboratory assessment and management. Hematology Am Soc Hematol Educ Program 2007.127-35. [Crossref] [PubMed]
- Piazza G. Thrombophilia testing, recurrent thrombosis, and women's health. Circulation 2014;130:283-7. [Crossref] [PubMed]
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295-306. [Crossref] [PubMed]
- Tincani A, Andreoli L, Chighizola C, et al. The interplay between the antiphospholipid syndrome and systemic lupus erythematosus. Autoimmunity 2009;42:257-9. [Crossref] [PubMed]
- Pons-Estel GJ, Andreoli L, Scanzi F, et al. The antiphospholipid syndrome in patients with systemic lupus erythematosus. J Autoimmun 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Shoenfeld Y, Meroni PL, Toubi E. Antiphospholipid syndrome and systemic lupus erythematosus: are they separate entities or just clinical presentations on the same scale? Curr Opin Rheumatol 2009;21:495-500. [Crossref] [PubMed]
- Ünlü O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol 2016;3:75-84. [Crossref] [PubMed]
- Kearon C, Julian JA, Kovacs MJ, et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008;112:4432-6. [Crossref] [PubMed]
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010;121:1706-12. [Crossref] [PubMed]
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007;92:199-205. [Crossref] [PubMed]
- Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009;301:2472-85. [Crossref] [PubMed]
- Smith TW, Pi D, Hudoba M, et al. Reducing inpatient heritable thrombophilia testing using a clinical decision-making tool. J Clin Pathol 2014;67:345-9. [Crossref] [PubMed]


