Drug-eluting stent restenosis treatment: an “old” stent, a “new” balloon or a “newer” scaffold?
The worldwide recurrent use of drug-eluting stent (DES), well explains that when the failure of this device occurs, namely in-stent restenosis (ISR), has great interest and impact on the daily clinical practice.
To establish the exact incidence of restenosis overall is not easy, depending on a number of different factors and variables. Novel generation of DES has reduced restenosis to numbers <10% (1-3).
Although the latest European guidelines on myocardial revascularization (4) have underlined how the use of drug-coated balloon (DCB) should be considered as “class I of recommendation, level of evidence A” for all types of ISR as well as the latest generation DES, the optimal treatment for DES-ISR remains uncertain due to different etiologies and treatment options that cause a still open debate.
Considering that DES are used for hard settings in cardiac catheterization laboratories worldwide, it is well intelligible that this issue has great interest and impact on the daily clinical practice in view of the everlasting battle between “balloonists” and “stentists”, namely DCB vs. DES strategy supporters for ISR treatment, respectively.
Focusing on the results of the RIBS IV study (5), in which 309 patients were treated with the latest-generation everolimus-eluting stent (EES) (n=155) has obtained better clinical results compared with first generation DCB (n=154). Clinical endpoints, composite of myocardial infarction (MI), cardiac death (CD) and target vessel revascularization (TVR) was highly reduced in the arm of EES therapy (10% vs. 18%; P=0.04; HR, 0.58; 95% CI: 0.35–0.98), thanks to a lower TVR (8% vs. 16%; P=0.035) as well as angiography findings. In fact, EES arm strategy had a relevant larger minimal lumen diameter (MLD) (2.03±0.7 vs. 1.80±0.6 mm; P<0.01) (absolute mean difference: 0.23 mm; 95% CI: 0.07–0.38), net lumen gain (LG) (1.28±0.7 vs. 1.01±0.7 mm; P<0.01), and a lower diameter stenosis (DS) rate (23%±22% vs. 30%±22%; P<0.01) and binary restenosis (BR) rate (11% vs. 19%; P=0.06). This could be an issue, since all available DCBs have paclitaxel as the active drug but is well demonstrated that coating technology and release method make some relevant differences. In effect, it is now well-known that a “class effect” for DCB does not exist and the excipient-based coating may heavily change the outcome from a clear success to failure. By our point of view, further trials are needed with a newer generation paclitaxel-coated balloon (PCB) employment for comparison against newer DES in this complex setting.
Another food for thought comes from Basavarajaiah et al. (6) study in which DCB was compared with second-generation DES for the treatment of DES restenosis. In this study, 247 patients were enrolled, corresponding to 302 DES-ISR, and were treated with DCB (81 patients; 104 lesions) or second-generation DES (166 patients; 198 lesions), respectively. At 12-month follow-up, there were no significant differences in the MACE rates (12.3% vs. 8.4%; P=0.3) and TLR rates (9.9% vs. 7.8%; P=0.6) between the two groups. However, it is to underline that a higher number of diabetics was in the DCB group (DCB 47% vs. DES 33%; P=0.03) and this pathological substrate usually speeds restenosis but thanks to the efficacy of DCB, it didn’t have its usual acceleration capabilities.
In their study, Habara et al. (7) compared DCB with DES, either paclitaxel or limus-eluting (i.e., sirolimus, everolimus), for DES restenosis (both the first and second-generation).
In this experience, 683 patients (777 lesions) were treated with DCB (306 lesions) or DES (471 lesions) at the discretion of the interventionist. The outcome at 6 to 8 months showed a recurrent restenosis rate of 23.5% in the DCB vs. 25.9% in the DES group (P=0.48). TLR was 15.7% vs. 20.3% respectively (P=0.13) and late lumen loss as well as BR resulted improved in the DCB group (respectively 0.34±0.57 vs. 0.68±0.76 mm, P<0.001, and 23.5% vs. 25.9%, P=0.48). The 12-month clinical outcome showed similar MACE rates (16.7% vs. 20.1%, P=0.27). There were no significant differences in terms of BR, TLR, and MACE between the two groups following the Cox regression analysis with propensity score adjustment suggested. Furthermore, it seems interesting to underline a favorable trend concerning to BR and TLR in DCB arm in non-focal type and bifurcation lesions.
Here we want to stress that, in the two studies, the same DCB was employed but different results were obtained.
Furthermore, also meta-analysis result disagrees, depending on studies selected. With these premises, we would like to suggest that a meta-analysis is like a good dish: it strongly depends on quality ingredients. In fact, a recent meta-analysis by Siontis and colleagues (8) have reported an interesting network meta-analysis comparing various treatment strategy for ISR, both bare metal stent (BMS) and DES, running from brachytherapy to latest DES available (i.e., EES). This study included 27 trials published from 2001 to 2014. According to the percent DS at angiographic follow-up (primary endpoint), the most effective treatment resulted in EES, with a difference of −9.0% (95% CI: −15.8%–2.2%) vs. DCB, namely the second-ranked. Moreover, EES was the best choice in the view of secondary endpoints, i.e., BR and TLR and DCB was second-ranked.
On the other hand, Lee and colleagues (9) reported another meta-analysis results in which DCB showed better outcomes than DES. In fact, according to the results, the risk of MACE, mostly TLR, was greatly lower in the DCB and DES (OR: 0.28; 95% CI: 0.14–0.53) than in the POBA group, with no relevant differences between the DCB and DES arms (OR: 0.84; 95% CI: 0.45–1.50). Based on the best treatment probability ranking the DCB appeared the better choice with 59.9%, second DES with 40.1%, and 0.1% for POBA in terms of TLR, whereas it was 63.0% (DCB), 35.3% (POBA), and 1.7% (DES) in terms of MI.
As we can see, final results change drastically with or without RIBS IV inclusion, respectively.
Bajraktari et al. (10) meta-analysis included latest Habara’s group results along with all randomized and observational studies that compared DEB with DES in patients with DES-ISR, for a total of 2052 cases. MACE [relative risk (RR) =1.00; 95% CI: 0.68–1.46; P=0.99), TLR (RR =1.15; 95% CI: 0.79–1.68; P=0.44), ST (RR =0.37; 95% CI: 0.10–1.34; P=0.13), MI (RR =0.97; 95% CI: 0.49–1.91; P=0.93) and CD (RR =0.73; 95% CI: 0.22–2.45; P=0.61) had no significant differences between DEB and DES treatment. Although, DCB group has showed a lower incidence for all-cause mortality (RR =0.45; 95% CI: 0.23–0.87; P=0.019) particularly in comparison to the first-generation DES (RR =0.33; 95% CI: 0.15–0.74; P=0.007). These results showed that DCB and DES have similar safety and efficacy for the DES-ISR treatment.
Here we would like to do further consideration: an intriguing approach for ISR treatment could be represented by the biovascular resorbable scaffold (BVS).
In this light, Moscarella et al. (11) study further strengthens our theory: it shows a prospective analysis on 116 patients who underwent a BVS implantation due to ISR. Mostly ISR lesions were DES restenosis (78, 61.6%), de novo ISR (92, 72.4%), and diffuse ISR (81, 63.8%). All patients (100%) have achieved procedural success. No in-hospital death, MI or revascularization occurred. At 15 months of follow-up, the incidence of the device-oriented composite end point (DOCE) estimated with the Kaplan-Meier method was 9.1%. No significant differences between DES and BMS restenosis groups were reported in terms of DOCE (10.9% vs. 6.4%; HR, 1.7; 95% CI: 0.5–6.5; P=0.425) and its singular components (CD: 2.8% vs. 2.0%; HR, 1.3; 95% CI: 0.1–14.1; P=0.843; target vessel MI: 1.5% vs. 0%; P=0.421; ischemia-driven TLR: 9.6% vs. 4.4%; HR, 2.3; 95% CI: 0.5–10.8; P=0.309).
These results are similar to results of Habara et al. study, in which ISR was treated with DCB. However, the use of BVS is limited by the BVS struts thickness, especially in small vessels with multiple stent layers already implanted, by the presence of this structure inside the vessel for at least 36–48 months and by the need of long DAPT (1 month for DCB vs. 12 months for BVS or 15 days for DCB vs. 6 months for BVS, in some cases).
Table 1 shows the most prominent studies here reported.
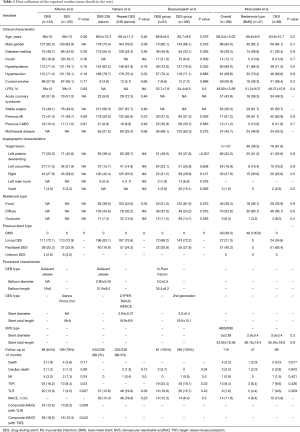
Full table
Indeed, not less important advantages come from the DCB use that interventionist should think of: (I) a shorter dual-antiplatelet therapy until 15 days (12) very useful and safe mostly for high-risk bleeding patients (13), on the contrary, a longer period is needed with currently available DES (4); (II) avoid the “onion-skin” phenomenon, caused by the apposition of a new metal layer to a previously implanted DES; (III) the great advantage of “leaving nothing behind” approach. In this view, DCB can be used several times for recurrent restenosis even if the previous DCB failed, without eternal prosthesis such avoiding the higher incidence of late/very late thrombotic events (14); (IV) notably, compared to stents, they share a greater deliverability in such complex lesions as well as long lesions that could be treated with a single device eluting antiproliferative drug and without a number of the permanent prosthesis, DCB warrants homogeneous distribution of the drug with a high concentration at the time of delivery and fast disappearance; (V) not secondary appear the cost-effectiveness of DCB for ISR treatment. In fact, DCB angioplasty is the least costly and most effective choice (15). In the actual period of spending review for public health, the option to save up to 34% (P<0.001) of 1-year global cost for ISR treatment using DCB strategy rather than repeated DES appears to be feasible and useful (16).
In conclusion, in our opinion and according to Bajraktari et al.’s meta-analysis and Moscarella et al.’s experience, “less is more”.
In this view, DCB might always represent the first line choice for the ISR treatment, it could be a simple way to reduce costs, the duration of DAPT (and subsequently lower risk of bleeding, especially for high risk patients) and, last but not least, it would avoid an eternal metal device.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Taniwaki M, Stefanini GG, Silber S, et al. 4-year clinical outcomes and predictors of repeat revascularization in patients treated with new-generation drug-eluting stents: a report from the RESOLUTE All-Comers trial (A Randomized Comparison of a Zotarolimus-Eluting Stent With an Everolimus-Eluting Stent for Percutaneous Coronary Intervention). J Am Coll Cardiol 2014;63:1617-25. [Crossref] [PubMed]
- Cassese S, Byrne RA, Tada T, et al. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart 2014;100:153-9. [Crossref] [PubMed]
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28-e292. [Crossref] [PubMed]
- Authors/Task Force members., Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]
- Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, et al. A Prospective Randomized Trial of Drug-Eluting Balloons Versus Everolimus-Eluting Stents in Patients With In-Stent Restenosis of Drug-Eluting Stents: The RIBS IV Randomized Clinical Trial. J Am Coll Cardiol 2015;66:23-33. [Crossref] [PubMed]
- Basavarajaiah S, Naganuma T, Latib A, et al. Treatment of drug-eluting stent restenosis: Comparison between drug-eluting balloon versus second-generation drug-eluting stents from a retrospective observational study. Catheter Cardiovasc Interv 2016;88:522-528. [Crossref] [PubMed]
- Habara S, Kadota K, Kanazawa T, et al. Paclitaxel-coated balloon catheter compared with drug-eluting stent for drug-eluting stent restenosis in routine clinical practice. EuroIntervention 2016;11:1098-105. [Crossref] [PubMed]
- Siontis GC, Stefanini GG, Mavridis D, et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet 2015;386:655-64. [Crossref] [PubMed]
- Lee JM, Park J, Kang J, et al. Comparison among drug-eluting balloon, drug-eluting stent, and plain balloon angioplasty for the treatment of in-stent restenosis: a network meta-analysis of 11 randomized, controlled trials. JACC Cardiovasc Interv 2015;8:382-94. [Crossref] [PubMed]
- Bajraktari G, Jashari H, Ibrahimi P, et al. Comparison of drug-eluting balloon versus drug-eluting stent treatment of drug-eluting stent in-stent restenosis: A meta-analysis of available evidence. Int J Cardiol 2016;218:126-35. [Crossref] [PubMed]
- Moscarella E, Ielasi A, Granata F, et al. Long-Term Clinical Outcomes After Bioresorbable Vascular Scaffold Implantation for the Treatment of Coronary In-Stent Restenosis: A Multicenter Italian Experience. Circ Cardiovasc Interv 2016;9:e003148. [Crossref] [PubMed]
- Cortese B, Berti S, Biondi-Zoccai G, et al. Drug-coated balloon treatment of coronary artery disease: a position paper of the Italian Society of Interventional Cardiology. Catheter Cardiovasc Interv 2014;83:427-35. [Crossref] [PubMed]
- Miglionico M, Mangiacapra F, Nusca A, et al. Efficacy and Safety of Paclitaxel-Coated Balloon for the Treatment of In-Stent Restenosis in High-Risk Patients. Am J Cardiol 2015;116:1690-4. [Crossref] [PubMed]
- Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur Heart J 2015;36:3320-31. [Crossref] [PubMed]
- Dorenkamp M, Boldt J, Leber AW, et al. Cost-effectiveness of paclitaxel-coated balloon angioplasty in patients with drug-eluting stent restenosis. Clin Cardiol 2013;36:407-13. [Crossref] [PubMed]
- Park K, Kim TE, Park KW, et al. Analysis of potential cost-savings after introduction of drug-eluting balloon angioplasty for in-stent restenosis or small vessel disease. Korean Circ J 2011;41:705-11. [Crossref] [PubMed]


