Calcification of arteries supplying the gastric tube increases the risk of anastomotic leakage after esophagectomy with cervical anastomosis
Introduction
Improvements in surgical techniques and perioperative care have led to a steady decrease in postoperative mortality of esophgectomy over the years (1). Anastomotic leakage, a radiologically or clinically apparent esophagogastrostomy anastomotic dehiscence and one of the major complications of esophagectomy, is an important cause of perioperative morbidity and mortality. Thoracic and cervical anastomosis is the two main methods used for upper gastrointestinal tract reconstruction. In Chinese population, the intrathoracic anastomotic leakage rate is reported to be 2.8% to 6.6% while the rate of cervical anastomotic leakage is 7.9% to 20.7% (2,3). A relatively higher incidence may result in an increased risk of stricture and perioperative death (4,5).
According to the results of related studies, risk factors of anastomotic leakage after esophagectomy include older age, ischemia of the gastric tube, malnutrition, hypotension, hypoxemia, renal insufficiency, neoadjuvant therapy, steroid use, smoking, diabetes, chronic obstructive pulmonary disease (COPD), cardiovascular disease, high body mass index, surgical approach, and the small number of esophagectomy procedures performed in a hospital (4,6-8). In all these factors, poor tissue perfusion is considered a major cause of anastomotic leakage.
Atherosclerosis, a known cause of ischemia, has been reported to compromise the blood supply of the gastric tube and cervical anastomosis (9). It has also been reported that calcification of the aortic wall and supra-aortic arteries can be used to predict cardiovascular events (10). Another study in European patients showed that calcification of the arteries supplying the gastric tube was independently associated with leakage after esophagectomy with cervical anastomosis (11). However, according to our knowledge, whether calcification of the gastric tube supplying arteries leads to a higher risk of leakage after esophagectomy with cervical anastomosis in Chinese esophageal cancer patients has not been investigated. In China, esophageal squamous cell carcinoma (ESCC) is the dominant subtype of esophageal cancer, which is different from western countries, where esophageal adenocarcinoma (EAC) is most commonly observed (12). Additionally, Asians have different adipose contents, partitioning, adipocyte morphology, and greater predisposition to cardiometabolic disease than Caucasians (13). Thus, the association between artery calcification and anastomotic leak for Chinese esophageal cancer patients requires further research.
In the current study, we retrospectively summarized the clinical characteristics of esophageal cancer patients who had received esophagectomy with cervical anastomosis and investigated leakage related factors. Especially, we aimed to identify the association between calcification of the supplying arteries of the gastric tube and anastomotic leakage in Chinese patients.
Methods
Patients and clinical data
The clinical data of 709 patients who had received elective esophagectomy with cervical anastomosis between January 2010 and May 2015 in Cancer Institute and Hospital, Chinese Academy of Medical Sciences (CAMS) were collected for the study. The demographic, clinical, and pathological features were obtained through reviewing their medical records. All patients were confirmed to have primary esophageal cancer by two independent pathologists (XL-F and SS-S). All the patients had received an abdominal-thoracic computed tomography (CT) scan and upper gastrointestinal endoscopic examination before surgery. TNM stages of the patients were determined according to the seventh edition of the Union for International Cancer Control (UICC) staging system. This study had been approved by the ethics committee of Cancer Institute and Hospital, CAMS and written informed consent was obtained from all patients before surgery.
Surgical procedures
Esophagectomy was performed via the left chest, or McKeown approach via the right chest with a cervical anastomosis in all patients. Slender conduit (3–5 cm Width) was used in 477 patients, and whatever kind of conduit was used for the other 232 patients. All the patients obtained cervical anastomosis. For better exposition of the operative field, the patient was took a supine position and placed with an extended neck and head turned to the right side. Through an oblique incision of the skin and the muscles on the left side of the neck, the cervical esophagus was mobilized, after which the specimen was removed through the cervical incision, with which the gastric tube was pulling up to the neck. An end-to-side esophagogastric anastomosis was performed in the neck using either the single-layer hand-sewn or circular stapled anastomotic technique by using an end-to-end anastomosis (EEA) stapler (DST SeriesTM EEATM 25 mm Stapler, 4.8 mm staple size; Covidien).
Postoperative care
After surgery, enteral nutrition was given on the postoperative day (POD) 1 to 3 through a jejunal feeding tube. The amount of enteral nutrition was gradually increased if the patient did not complain nausea, vomiting, abdominal distension, abdominal pain or diarrhea. The drainage in the neck was usually removed on POD 3 to POD 5. Without postoperative complications, oral feeding started around POD 7 to POD 9. Intravenous proton pump inhibitors (PPIs) or H2-blockers were usually prescribed on POD 1 and were given orally after oral feeding. The majority of patients were discharged between POD 11 and POD 13. All the patients were followed up 2 weeks after discharge, and every 3 months afterwards.
Diagnosis of anastomotic leakage
A cervical anastomotic leakage was first suspected if any clinical signs of leakage (e.g., fever or presence of purulent or salivary discharge from cervical wound or chest tube), radiologic signs of leakage (e.g., contrast leakage or fluid and air levels surrounding the anastomosis), or signs of anastomotic dehiscence during endoscopy or reoperation appeared during the follow-up period. In case anastomotic leakage was clinically suspected, a CT scan, water-soluble contrast swallow study or endoscopy was performed. No routine diagnostic tests were performed (14).
Imaging acquisition and assessment
A visual calcification grading system was modified from a previously reported score for grading aortic wall abnormalities in the prediction of cardiovascular events in our study. Considering whether such a system can be applied to the Chinese population is still uncertain. Only the presence or absence of calcification in vessels was considered in our study. Due to the lack of the imaging data of 36 of the 709 patients enrolled, CT imaging of 673 patients who underwent preoperative chest and abdominal CT examinations were eventually included to be analyzed. All preoperative CT studies were independently reviewed by two experienced thoracic surgeons (B-Q and JG-L, with 10 and 9 years of experience performing thoracic surgery, respectively) who were blinded to patient and operation related characteristics and clinical outcome in terms of anastomotic leakage. To ensure the reliability of this study, another radiologist (J-J), who was also blinded to the data of patients, was also invited to evaluate CT imaging. The selection of investigated vessels was based on anatomic studies, which showed that the right gastroepiploic artery exclusively supplies the gastric tube and originates from the aorta via the celiac axis, common hepatic artery, and gastroduodenal artery (9,15). Furthermore, calcifications in the splenic and left gastroepiploic arteries were assessed because it has been reported that about 20% of the gastric tube is supplied by the left gastroepiploic arteries before divided (15). To facilitate the evaluation the calcification and ensure the accuracy of the results, we divided the vessels mentioned into four parts: aorta (i.e., descending part of thoracic aorta and abdominal part of aorta above celiac level), celiac axis, right postceliac arteries (i.e., common hepatic artery, gastroduodenal artery, and right gastroepiploic artery) and left postceliac arteries (i.e., splenic artery and left gastroepiploic artery). Calcifications in the vessels were judged in the transverse plane (reconstruction thicknesses and intervals were 5 mm and 5 mm, respectively) and were reviewed with the standard mediastinal window (window width, 300–400 HU; window level, 40–50 HU). Discrepancies in interpretation between observers were resolved by consensus.
Statistical analysis
Continuous variables were presented with mean and standard deviation. Frequencies and percentages were presented by categorical variables. Continuous variables were compared between groups with Wilcoxon two-sample test while categorical variables by Chi-square test. Variables were entered in a multivariable logistic regression model to evaluate whether these factors were independently associated with the occurrence of anastomotic leakage if they were associated to the cervical anastomotic leak in the univariate analysis. Probability values of less than 0.05 were considered to be statistically significant, and there was no adjustment made for multiplicity of comparisons.
Results
A total of 709 patients in our study underwent esophagectomy with cervical anastomosis, among which 17.2% (122 of 709) had postoperative anastomotic leakage. The average length of hospital stay after surgery was 13.05 days for patients without anastomotic leakage and 34.85 days for those with anastomotic leakage (P<0.0001). Postoperative 30-day mortality rate was 4.92% (6 of 122) and 0.34% (2 of 587) for patients with and without anastomotic leakage, respectively (P=0.0010) (Table 1).
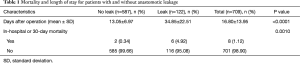
Full table
Comparison of demographic and clinical characteristics of patients with or without leakage (Table 2) showed that American society of Anesthesiologists (ASA) risk class, prior thoracic surgery, upper digestive tract ulcer, COPD, hypertension, peripheral vascular disease, renal insufficiency, forced expiratory volume in one second (FEV1) predicted and diffusion capacity for carbon monoxide of the lung (DLCO) predicted were significantly associated with an increased risk of anastomotic leakage (P<0.05).
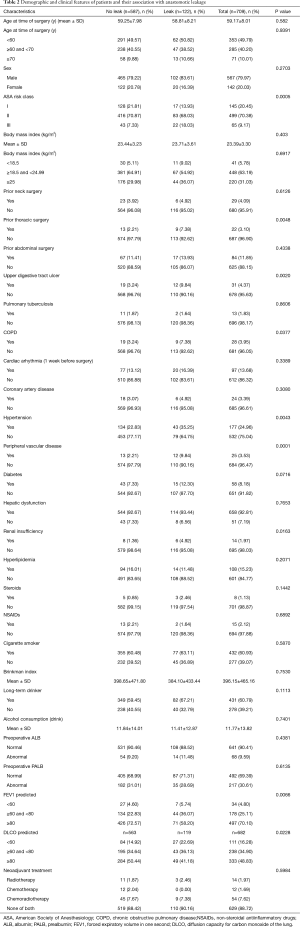
Full table
We next evaluated the effect of surgical and pathological factors on anastomotic leakages. The results of the comparison showed that none of the surgical (Table 3) or pathological features (Table 4) had significant association with cervical anastomotic leakage.
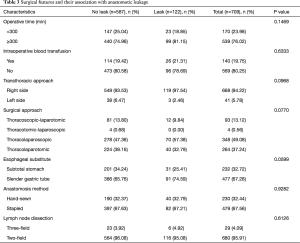
Full table
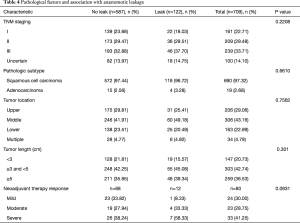
Full table
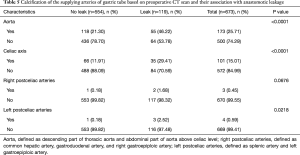
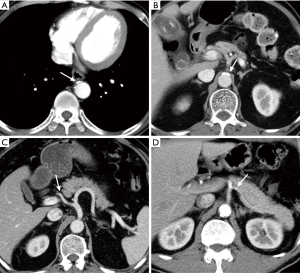
Further, we investigated whether calcification of gastric tube supplying arteries would affect an anastomotic leakage in the 673 patients with preoperative CT scan. The proportion of patients with calcification in the aorta, celiac axis, right postceliac arteries and left postceliac arteries were 25.71% (173 of 673), 15.01% (101 of 673), 0.45% (3 of 673) and 0.59% (4 of 673), respectively. Chi-square test revealed that patients with calcification of aorta (P<0.0001), celiac axis (P<0.0001) and left postceliac arteries (P=0.0218) had significantly higher rate of anastomotic leakage in unvariate analysis. No significant increased risk of anastomotic leak was discovered for calcification of the right postceliac arteries (Table 5). Examples of the image characteristics are presented in Figure 1.
Full table
To define independent risk factors of postoperative cervical anastomotic leak for patients who underwent esophagectomy, the calcification of the aorta, celiac axis, and left postceliac arteries were entered into the multivariate logistic regression model, along with ASA risk class, prior thoracic surgery, upper digestive tract ulcer, pulmonary tuberculosis, hypertension, peripheral vascular disease, renal insufficiency, FEV1 predicted and DLCO predicted. The results of the analysis showed that the upper digestive tract ulcer (OR =2.666; 95% CI: 1.128–6.300; P=0.0255), peripheral vascular disease (OR =2.963; 95% CI: 1.166–7.530; P=0.0225), renal insufficiency (OR =3.686; 95% CI: 1.117–12.169; P=0.323), ASA risk class (OR =1.725; 95% CI: 1.098–2.710; P=0.0180), calcifications of aorta (OR =2.300; 95% CI: 1.429–3.704; P=0.0006) and celiac axis (OR =1.823; 95% CI: 1.051–3.162; P=0.326) were independently associated with increased risk of anastomotic leak (Table 6).
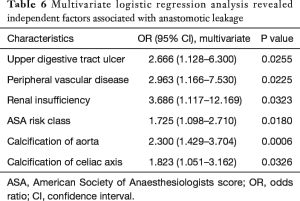
Full table
Discussion
The assessment of calcification in our study was based on a previously described and validated visual grading system used to score aortic abnormalities (e.g., calcifications, irregularity, plaques, and elongation of the aortic wall) observed on routine diagnostic CT images that was shown to be useful in the prediction of cardiovascular events (10,16). However, besides calcifications, the addition of these parameters did not lead to substantial improvement of the prediction model (10). Furthermore, scores for calcifications of the thoracic aorta can be used to predict the incidence rate of cardiovascular events (10). According to these studies, another recent study focused on the calcification of the arteries supplying the gastric tube and showed that a higher score of calcification in the aorta and right postceliac arteries conferred a higher chance of cervical anastomotic leakage (11). Because Chinese patients are different from those of the western countries in terms of pathological subtype and other biochemical factors (12,13), it was uncertain whether a quantitative system could be used in the study of Chinese people. We decided to use a qualitative research method to assess the presence or absence of calcifications in feeding arteries of the gastric tube. Fortunately, through the qualitative analysis of the image data of 673 patients, a similar result to European researchers was obtained, and we confirmed that calcification of the arterial supply of the gastric tube detected on routine preoperative CT images is esophagectomy in Chinese patients.
Pathophysiologically, the relationship between artery calcification and leakage is rational because the right gastroepiploic artery originating from this trajectory mainly supplies the gastric tube and anastomosis. Although the calcification in the right postceliac arteries, especially in the right gastroepiploic artery, may compromise the local perfusion of the gastric tube, different from the research based on European population (11), calcification in the right postceliac arteries was not significantly related to the leakage in the our final results. Compared with the previous study (11), we noticed that the proportion of calcification in the right postceliac arteries so small in our study (3 of 673 vs. 11 of 246) that it was difficult to make a conclusion with such a small number of patients. Calcification of the celiac axis, whose blood flow in the right gastroduodenal artery comes from, may also compromise the perfusion of gastric tube indirectly. The relation between the leakage and the calcification of the celiac axis was confirmed in our research. However, studies focused on Europeans did not show a similar result (11,17). We speculated that the difference in the distribution and extent of the calcification between Western and Chinese population may finally result in different results. Thus, a larger sample size and further research may be necessary to clarify the difference.
Tissue ischaemia as a potential mechanism for anastomotic leakage is likely to be moderated by a combination of generalized vascular disease (marked by peripheral vascular disease) and compromised local perfusion (marked by calcification of celiac axis). In addition, anastomotic leakage has also been found to be related to congestion due to insufficient venous drainage, the method of anastomosis construction, the width of the gastric tube, mechanical tension, and poor nutrition (18-20). Regarding these factors, various attempts to optimize the conditions of the anastomosis have been reported. Related potential risk factors such as surgical procedures and pathological factors were also considered in our study, yet were not found to be associated in this cohort of patients. However, it is worth noting that some new cervical anastomosis methods, such as embedded three-layer anastomosis, hybrid-layered suture in hand sewn EEA, and cervical end-to-side triangulating anastomosis have significantly decreased the incidence of cervical anastomotic leakage (21-23).
It is worth mentioning that our qualitative assessment of artery calcification is simplified method. Although the extent of calcification was not assessed, the presence of calcification in arteries could still remind the surgeons which patient had a higher chance to have a cervical leakage so that more adequate preparation before surgery, more carefully in the operation, and better nutritional support in postoperative treatment could be provided. Thus, a quantitative visual grading system with good practicability and reproducibility, which is established to help surgeons to screen out patients with higher risk of anastomotic leakage will be helpful. Furthermore, a quantitative system may also help surgeons to decide whether a patient needs to take a radiological examination before serious clinical symptoms appear so that a minor anastomotic leakage could be treated timely and correctly. With more studies validating the reliability of the association between calcification and cervical anastomotic leakage, this quantitative method could be potentially promoted in clinical practice.
There are a few limitations in our study. Firstly, this study was confined to a population that underwent elective esophagectomy with cervical anastomosis. Outcomes might be different in populations that undergo other surgical approaches. Secondly, artery calcification does not always lead to impaired perfusion, and there are more specific ways to determine the extent of vascular disease and local perfusion, such as use of laser Doppler flowmetry or CT angiography of the abdomen, which were not applied in the study. Furthermore, no prospective data are yet available to prove the clinical benefit of the calcification evaluation in reducing morbidity, and a well-designed quantitative study needs to be carried out.
Conclusions
In this study, we found that vascular calcifications of the arteries that supply the gastric tube were significant risk factors of cervical anastomotic leakages. Further study with a larger sample size and a quantitative or semiquantitative model of calcification assessment should be carried out to confirm the findings and to optimize a risk prediction model for anastomotic leakage.
Acknowledgements
Funding: This study was supported in part by grants from Beijing Hope Marathon Foundation of Cancer Foundation of China (No. LC2015L17).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics committee of Cancer Institute and Hospital, CAMS (NCC2014RE-001) and written informed consent was obtained from all patients.
References
- Lerut T, Coosemans W, Decker G, et al. Anastomotic complications after esophagectomy. Dig Surg 2002;19:92-8. [Crossref] [PubMed]
- Chen C, Yu Z, Jin Q, et al. Clinical features and risk factors of anastomotic leakage after radical esophagectomy. Zhonghua Wai Ke Za Zhi 2015;53:518-21. [PubMed]
- Zhang L, Li H, Hou SC, et al. An aggregate score system to stratify the risk of anastomotic leakage after esophageal carcinoma surgery. Chin J Thorac Surg 2016;3:15-20. (Electronic Edition).
- Atkins BZ, Shah AS, Hutcheson KA, et al. Reducing hospital morbidity and mortality following esophagectomy. Ann Thorac Surg 2004;78:1170-6; discussion 1170-6. [Crossref] [PubMed]
- Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg 2004;10:71-5. [PubMed]
- Davies N. Surgeon volumes in oesophagogastric and hepatopancreatobiliary resectional surgery. Br J Surg 2011;98:891-3. [Crossref] [PubMed]
- Zhang SS, Yang H, Luo KJ, et al. The impact of body mass index on complication and survival in resected oesophageal cancer: a clinical-based cohort and meta-analysis. Br J Cancer 2013;109:2894-903. [Crossref] [PubMed]
- Kassis ES, Kosinski AS, Ross P Jr, et al. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013;96:1919-26. [Crossref] [PubMed]
- Ndoye JM, Dia A, Ndiaye A, et al. Arteriography of three models of gastric oesophagoplasty: the whole stomach, a wide gastric tube and a narrow gastric tube. Surg Radiol Anat 2006;28:429-37. [Crossref] [PubMed]
- Gondrie MJ, Mali WP, Jacobs PC, et al. Cardiovascular disease: prediction with ancillary aortic findings on chest CT scans in routine practice. Radiology 2010;257:549-59. [Crossref] [PubMed]
- van Rossum PS, Haverkamp L, Verkooijen HM, et al. Calcification of arteries supplying the gastric tube: a new risk factor for anastomotic leakage after esophageal surgery. Radiology 2015;274:124-32. [Crossref] [PubMed]
- Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol 2009;24:729-35. [Crossref] [PubMed]
- Haldar S, Chia SC, Henry CJ. Body Composition in Asians and Caucasians: Comparative Analyses and Influences on Cardiometabolic Outcomes. Adv Food Nutr Res 2015;75:97-154. [Crossref] [PubMed]
- Boone J, Rinkes IB, van Leeuwen M, et al. Diagnostic value of routine aqueous contrast swallow examination after oesophagectomy for detecting leakage of the cervical oesophagogastric anastomosis. ANZ J Surg 2008;78:784-90. [Crossref] [PubMed]
- Liebermann-Meffert DM, Meier R, Siewert JR. Vascular anatomy of the gastric tube used for esophageal reconstruction. Ann Thorac Surg 1992;54:1110-5. [Crossref] [PubMed]
- Jacobs PC, Prokop M, Oen AL, et al. Semiquantitative assessment of cardiovascular disease markers in multislice computed tomography of the chest: interobserver and intraobserver agreements. J Comput Assist Tomogr 2010;34:279-84. [Crossref] [PubMed]
- Goense L, van Rossum PS, Weijs TJ, et al. Aortic Calcification Increases the Risk of Anastomotic Leakage After Ivor-Lewis Esophagectomy. Ann Thorac Surg 2016;102:247-52. [Crossref] [PubMed]
- Walther B, Johansson J, Johnsson F, et al. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg 2003;238:803-12; discussion 812-4. [Crossref] [PubMed]
- Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg 1995;169:634-40. [Crossref] [PubMed]
- Dewar L, Gelfand G, Finley RJ, et al. Factors affecting cervical anastomotic leak and stricture formation following esophagogastrectomy and gastric tube interposition. Am J Surg 1992;163:484-9. [Crossref] [PubMed]
- Sun HB, Li Y, Liu XB, et al. Embedded Three-Layer Esophagogastric Anastomosis Reduces Morbidity and Improves Short-Term Outcomes After Esophagectomy for Cancer. Ann Thorac Surg 2016;101:1131-8. [Crossref] [PubMed]
- Feng F, Sun L, Xu G, et al. Albert-Lembert versus hybrid-layered suture in hand sewn end-to-end cervical esophagogastric anastomosis after esophageal squamous cell carcinoma resection. J Thorac Dis 2015;7:1917-26. [PubMed]
- Nakata K, Nagai E, Ohuchida K, et al. Outcomes of cervical end-to-side triangulating esophagogastric anastomosis with minimally invasive esophagectomy. World J Surg 2015;39:1099-104. [Crossref] [PubMed]


