Prognostic significance of red cell distribution width in elderly patients undergoing resection for non-small cell lung cancer
Introduction
The preoperative assessment of surgical risk is critical especially when surgery is considered for complicated and high-risk cases. Owing to recent advances in less-invasive surgery, anesthesia, and perioperative managements, surgery can be performed in those who have comorbidities and are of advanced age. In this background, identifying a reliable preoperative parameter that can predict postoperative outcome is of importance.
In recent years, red cell distribution width (RDW) has been revealed to be a prognostic indicator for various diseases. RDW represents the volume variation among red blood cells, and is calculated by the following equation: RDW (%) = standard deviation of red cell volume ÷ mean cell volume ×100. RDW has been traditionally used in the investigation of the etiology of anemia (1). The volume of erythrocytes is consistent, and RDW is normal in the case of anemia caused by rapid bleeding, whereas the increase of relatively large reticulocytes during the recovery process of anemia raises RDW in the case of slowly progressing anemia. RDW has recently been reported to also be an inflammatory indicator. There is mounting evidence linking elevated RDW and adverse outcomes in cardiac disease (2-4), pulmonary embolism (5), trauma (6), septic shock (7), acute pancreatitis (8), hepatitis (9), stroke (10), dementia (11), metabolic syndrome (12), and respiratory diseases such as non-small cell lung cancer (NSCLC) (13,14), chronic obstructive pulmonary disease (15), interstitial pneumonia (16) and sarcoidosis (17). High RDW has been associated with increased mortality and poor pulmonary function also in the general population (18-20).
The aim of this study was to investigate the prognostic impact of RDW on outcomes in elderly patients after surgery for NSCLC.
Methods
Patient cohort
A total of 1,061 patients with NSCLC who underwent resection at our institute between 1998 and 2012 were retrospectively analyzed. Before the study, the Research Review Board of Tokyo University examined and approved our research protocol in accordance with the Declaration of Helsinki (project approval No. 2406). All patients undergoing surgery irrespective of this study provided written informed consent for the review of their medical charts before the surgery. A total of 69 patients were excluded, because their records regarding RDW could not be obtained (n=8) or because they had evidence of a small-cell carcinoma component (n=8), carcinoid (n=4), distant metastases (n=7), dissemination (n=22), malignant effusion (n=9), or N3 disease (n=11).
Data extraction
The following 18 variables were investigated as prognostic factors: RDW (%), age, gender, smoking index (pack-years), white blood cell count (WBC, ×103/µL), neutrophil to lymphocyte ratio (NLR), hemoglobin (g/dL), platelet count (×104/µL), albumin (g/dL), C-reactive protein (CRP; mg/dL), carcinoembryonic antigen (CEA; ng/mL), %vital capacity (%VC; %), %forced expiratory volume in one second (%FEV1; %), histology, T factor, N factor, surgical approach and surgical procedures. Laboratory data were obtained within one month before surgery. All measurements were performed at a single laboratory and hematological parameters were analyzed using two automated hematology analyzers; Coulter Gen-S (Beckman Coulter) and Sysmex XE-2100 (Sysmex). The tumor stage was determined pathologically according to the seventh edition of the TNM staging system of the International Union Against Cancer (21), and the histologic tumor type was determined according to the third edition of the World Health Organization classification (22).
Outcomes
We evaluated morbidity, hospital mortality, and postoperative hospital stay as short-term outcomes and overall survival (OS) and disease-free survival (DFS) as long-term outcomes. Morbidity included prolonged air leakage (>7 days), bronchial fistula, pneumonia, pulmonary embolism, pyothorax, chylothorax, exacerbation of interstitial pneumonia, cardiovascular disease, arrhythmia, postoperative bleeding, reoperation, stroke and delirium. The OS was calculated from the date of surgery to the time of death. The DFS was measured from the date of surgery to the date of lung cancer recurrence or death from any cause. The interval between follow-up examinations was every 3 months for the first 2 years and every 4 months for up to 5 years. A full examination and a chest X-ray were performed at each visit, and a computed tomography (CT) scan was performed annually. Other investigations were performed when indicated.
Statistical analysis
Statistical analyses were performed using JMP 11 software (SAS Institute, Cary, NC, USA). The cutoff value of RDW was set at 13.8, which was the median value in the elderly patients (age ≥75 years). Another cutoff value of 15 was arbitrarily chosen. Comparisons of the clinicopathological features were analyzed using Student’s t-test, Welch’s method, or the χ2 test. The OS and DFS were estimated by the Kaplan-Meier method and were compared by the log-rank test. Risk factors for morbidity and recurrence were analyzed by multiple logistic regression analysis, and prognostic factors for OS and DFS were assessed by Cox’s proportional hazards regression model using the following binary variables: RDW (>13.8/≤13.8), sex (M/F), smoking (yes/no), WBC (>6×103/≤6×103/µL), NLR (>3/≤3), hemoglobin (<12/≥12 g/dL), platelet count (>20×104/≤20 ×104/µL), albumin (<3.5/≥3.5 g/dL), CRP (>0.5/≤0.5 mg/dL), CEA (>5/≤5 ng/mL), adenocarcinoma (yes/no), %VC (<80%/≥80%), %FEV1 (<80%/≥80%),T factor (T2–4/T1), N factor (N1–2/N0), approach (thoracoscopy/open) and sublobar resection (yes/no). Factors associated with higher levels of RDW were analyzed by multiple linear regression analysis using the following continuous and binary variables: age, gender (M/F), smoking index (pack-years), WBC, NLR, hemoglobin, platelet count, albumin, CRP, CEA, adenocarcinoma (yes/no), %VC, %FEV1, T factor (T2–4/T1), N factor (N1–2/N0) and preoperative comorbidities such as interstitial pneumonia (yes/no), cardiovascular disease (yes/no), cerebrovascular disease (yes/no), diabetes mellitus (yes/no), autoimmune disease (yes/no) and history of malignancy (yes/no). The relationship between RDW, hemoglobin and age was evaluated by calculation of the Pearson correlation coefficient.
Results
The clinicopathological data of 992 patients were analyzed. The median value of RDW was 13.5. The normal value of RDW was set at 12.0–15.9 in our institution. RDW was higher than 15.9 in 73 (7.4%) patients and lower than 12.0 in 5 (0.5%) patients. The morbidity, the 30-day mortality and the hospital mortality in the total cohort were 195 (20%), 1 (0.1%) and 11 (1.1%), respectively. The median follow-up time was 47 months. The recurrence of lung cancer was detected in 238 (24%) patients during follow-up.
In the study cohort, 275 (28%) cases were 75 years of age or older. The elderly patients were divided into two groups according to the RDW value. The relationships between RDW and other characteristics in the elderly patients were shown in Table 1. High RDW was significantly associated with low albumin, T stage >1 and morbidity. The 30-day and hospital mortality were 1 (0.8%) and 4 (3.0%) in the high RDW group and 0 and 1 (0.7%) in the low RDW group, respectively.
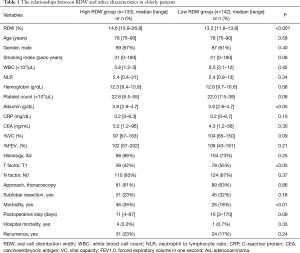
Full table
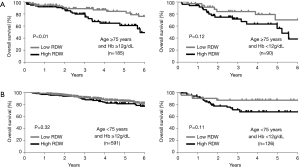
Multiple logistic regression analysis using the 17 variables listed in the Methods section revealed that high RDW was an independent risk factor for morbidity in the elderly patients [hazard ratio (HR) 2.1; P<0.01], whereas it was not in the younger patients (Table 2). The OS and DFS curves for high and low RDW are shown in Figure 1. The survival outcomes with high RDW were poorer than those with low RDW, especially in the elderly patients (5-year OS, 59% vs. 81%, P<0.01; 5-year DFS, 48% vs. 70%, P<0.01). Cox regression analysis revealed that high RDW was an independent prognostic factor in the elderly patients (OS: HR 2.1, P<0.01; DFS: HR 2.0, P<0.01), whereas it was not in the younger patients (Table 3). Multiple logistic regression analysis revealed that high RDW was also a risk factor for recurrence in the elderly patients (HR 2.0; P=0.01), but not in the younger patients.
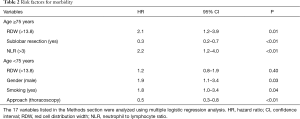
Full table
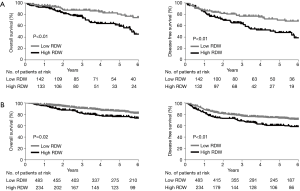
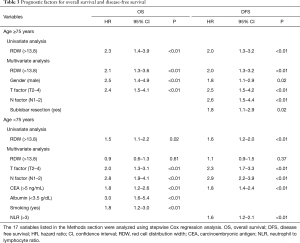
Full table
Figure 2 showed that there was little correlation between RDW and age (R =0.15; P<0.01). Especially in the elderly patients, RDW was revealed to be unaffected by age (R =0.01; P=0.86). Table 1 also showed that there was no significant difference in age between the high RDW group and the low RDW group. Factors associated with higher levels of RDW in the elderly patients were analyzed using multiple linear regression analysis using the 21 variables listed in the Methods section (Table 4). High RDW was associated with low hemoglobin, high NLR, histology other than adenocarcinoma, absence of diabetes mellitus and past history of malignancy. To eliminate the confounding bias of anemia, all analyses were repeated excluding the patients with anemia (hemoglobin <12, <11, or <10 g/dL), and the same results were obtained (data not shown). The correlation between RDW and hemoglobin was weaker in elderly patients (R =−0.27) than in younger patients (R =−0.49). The differences in the prognostic significance of RDW according to age and hemoglobin are shown in Figure 3. High RDW without anemia had little influence on prognosis of younger patients, but in elderly patients, elevated RDW without anemia was more prognostic than high RDW due to anemia.


Full table
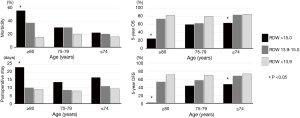
The outcomes of the subdivided groups according to age (≥80, 75–79, and ≤74 years) and RDW (>15, 13.9–15, and <13.9) are shown in Figure 4. In the patients aged 80 years or older (n=90), a RDW of >15 was significantly associated with high morbidity and long postoperative hospital stay. The prognosis of this highest-risk group (n=16) was extremely poor (5-year OS, 24%; 5-year DFS, 0%). This group had frequent preoperative comorbidities (81%; history of malignancy in ten cases, cardiovascular disease in four cases, and interstitial pneumonia in three cases), frequent complications (56%; pneumonia in five cases, pyothorax in one case, prolonged air leakage in one case, postoperative bleeding in one case, exacerbation of interstitial pneumonia in one case, stroke in one case, and delirium in one case) and a high risk of recurrence (56%).
Discussion
In this study, high RDW was found to be an independent risk factor for morbidity, OS, and DFS in elderly patients who underwent resection for NSCLC, whereas it was not in younger patients. The outcomes were extremely poor, especially in the highest-risk group (age ≥80 years and RDW >15).
The difference in the prognostic significance of RDW according to age has rarely been described. In our study, the correlation between RDW and adverse outcome was mostly due to anemia in younger patients. However, RDW is particularly worthy of measurement in elderly patients, because elevated RDW in the elderly had a stronger influence on prognosis in cases without anemia.
Although it has been demonstrated that higher RDW is associated with adverse outcomes in various diseases, the mechanism underlying this association remains unclear. The major causes of anisocytosis include iron deficiency anemia, megaloblastic anemia, immune hemolytic anemia, myelodysplastic syndrome, and liver disease. This suggests that high RDW is caused by systemic inflammation, nutritional deficiency, and bone marrow dysfunction. Larger variation of red blood cell volume is strictly associated with decreased erythrocyte deformability, which in turn impairs blood flow through microcirculation (23). Oxidative stress was also revealed to be involved in the association between elevated RDW and clinical endpoints (24,25). In our study, higher RDW was associated with low hemoglobin, high NLR, histology other than adenocarcinoma, past history of malignancy, and absence of diabetes mellitus in elderly patients. An association between diabetes mellitus and low RDW was also reported in a previous study (26).
The interesting characteristic of RDW was that high RDW seemed to represent not only increased surgical risk but also a potential hazard of recurrence of NSCLC. High RDW may be associated with impaired antitumor immunity, and that may explain why high RDW was closely associated with a past history of malignancy. Further work is needed to elucidate the exact mechanism by which RDW impacts prognosis.
RDW is a particularly useful prognostic indicator because of its cost-effectiveness, simplicity and predictive ability of both short- and long-term outcomes. RDW is a simple item included in usual complete blood counts and no additional examination, cost, calculation, or detailed medical history is required. Our results appear to be common among surgeries for other malignancies as well as NSCLC. The prognostic significance of RDW in other diseases should be evaluated in future studies.
There are some limitations to the present study. The study design was retrospective and observational, and this was a single-institution study. This study did not include the non-surgically treated patients, and therefore, the influence of selection bias should be considered. Our results can be applied only for surgically treated patients. An additional limitation is that serum levels of factors that can influence RDW (e.g., iron, vitamin B12, and folic acid) were not determined.
On the basis of the extremely poor outcomes observed for the highest-risk group (age ≥80 years and RDW >15) in our study, surgical indication for these patients should be carefully assessed and limited resection might be beneficial. Moreover, RDW should be taken into account when a clinical trial of surgical treatment for the elderly is designed. The role of preoperative RDW as a predictive factor needs to be further tested in a prospective study in the future.
In conclusion, high RDW appears to be a risk factor indicating postoperative morbidity and reduced survival in elderly patients with NSCLC. The results from this study should be further tested in a prospective manner so that surgical/medical oncologists can take this parameter into account when surgical intervention is considered in the elderly.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Before the study, the Research Review Board of Tokyo University examined and approved our research protocol in accordance with the Declaration of Helsinki (project approval No. 2406). All patients undergoing surgery irrespective of this study provided written informed consent for the review of their medical charts before the surgery.
References
- Sultana GS, Haque SA, Sultana T, et al. Value of red cell distribution width (RDW) and RBC indices in the detection of iron deficiency anemia. Mymensingh Med J 2013;22:370-6. [PubMed]
- Dabbah S, Hammerman H, Markiewicz W, et al. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol 2010;105:312-7. [Crossref] [PubMed]
- Balta S, Demir M, Kucuk U, et al. Red cell distribution width in patients with atrial fibrillation. J Intern Med 2014;275:545. [Crossref] [PubMed]
- Zöller B, Melander O, Svensson P, et al. Red cell distribution width and risk for venous thromboembolism: a population-based cohort study. Thromb Res 2014;133:334-9. [Crossref] [PubMed]
- Zorlu A, Bektasoglu G, Guven FM, et al. Usefulness of admission red cell distribution width as a predictor of early mortality in patients with acute pulmonary embolism. Am J Cardiol 2012;109:128-34. [Crossref] [PubMed]
- Balta S, Demirkol S, Akgul EO. Red blood cell distribution width is predictive of mortality in trauma patients. J Trauma Acute Care Surg 2013;75:345-6. [Crossref] [PubMed]
- Balta S, Demirkol S, Hatipoglu M, et al. Red cell distribution width is a predictor of mortality in patients with severe sepsis and septic shock. Am J Emerg Med 2013;31:989-90. [Crossref] [PubMed]
- Balta S, Demirkol S, Cakar M, et al. Red cell distribution width: a novel and simple predictor of mortality in acute pancreatitis. Am J Emerg Med 2013;31:991-2. [Crossref] [PubMed]
- Lou Y, Wang M, Mao W. Clinical usefulness of measuring red blood cell distribution width in patients with hepatitis B. PLoS One 2012;7:e37644. [Crossref] [PubMed]
- Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci 2009;277:103-8. [Crossref] [PubMed]
- Weuve J, Mendes de Leon CF, Bennett DA, et al. The red cell distribution width and anemia in association with prevalent dementia. Alzheimer Dis Assoc Disord 2014;28:99-105. [Crossref] [PubMed]
- Sánchez-Chaparro MA, Calvo-Bonacho E, González-Quintela A, et al. Higher red blood cell distribution width is associated with the metabolic syndrome: results of the Ibermutuamur CArdiovascular RIsk assessment study. Diabetes Care 2010;33:e40. [Crossref] [PubMed]
- Koma Y, Onishi A, Matsuoka H, et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One 2013;8:e80240. [Crossref] [PubMed]
- Balta S, Arslan Z, Unlu M, et al. The association between red cell distribution width and non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:954. [Crossref] [PubMed]
- Balta S, Aydogan M, Demirkol S, et al. Red cell distribution width: a novel and simple predictor of mortality in chronic obstructive pulmonary disease. COPD 2014;11:475-6. [Crossref] [PubMed]
- Nathan SD, Reffett T, Brown AW, et al. The red cell distribution width as a prognostic indicator in idiopathic pulmonary fibrosis. Chest 2013;143:1692-8. [Crossref] [PubMed]
- Ozsu S, Ozcelik N, Oztuna F, et al. Prognostic value of red cell distribution width in patients with sarcoidosis. Clin Respir J 2015;9:34-8. [Crossref] [PubMed]
- Perlstein TS, Weuve J, Pfeffer MA, et al. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med 2009;169:588-94. [Crossref] [PubMed]
- Patel KV, Semba RD, Ferrucci L, et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci 2010;65:258-65. [Crossref] [PubMed]
- Grant BJ, Kudalkar DP, Muti P, et al. Relation between lung function and RBC distribution width in a population-based study. Chest 2003;124:494-500. [Crossref] [PubMed]
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours: John Wiley & Sons, 2011.
- Travis WD, Brambilla W, Muller-Hermelink HK, et al. World Health Organization classification of tumors: Pathology and genetics of tumors of the lung, pleura, thymus and heart. Lyon: ICRC Press, 2004.
- Patel KV, Mohanty JG, Kanapuru B, et al. Association of the red cell distribution width with red blood cell deformability. Adv Exp Med Biol 2013;765:211-6. [Crossref] [PubMed]
- Zhao Z, Liu T, Li J, et al. Elevated red cell distribution width level is associated with oxidative stress and inflammation in a canine model of rapid atrial pacing. Int J Cardiol 2014;174:174-6. [Crossref] [PubMed]
- Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal 2008;10:1923-40. [Crossref] [PubMed]
- Engström G, Smith JG, Persson M, et al. Red cell distribution width, haemoglobin A1c and incidence of diabetes mellitus. J Intern Med 2014;276:174-83. [Crossref] [PubMed]


