Transapical beating-heart chordae implantation in mitral regurgitation: a new horizon for repairing mitral valve prolapse
Mitral regurgitation (MR) is increasingly prevalent in western countries despite reduced incidence of rheumatic disease (1,2). MR results from several heterogeneous conditions, including disorders of the valve leaflets, mitral annulus, chordae tendineae, papillary muscles and left ventricle (LV). MR causes are roughly classified as primary (i.e. organic/structural) or secondary (i.e. functional/non-structural) (3).
Degenerative disease is the most common cause of primary MR that often necessitates surgery. It involves mitral valve prolapse (Carpentier’s classification type II) and covers a large spectrum of lesions (isolated scallop to multi-segment prolapse). Prolapse location, valvular/annular calcifications, and severity of annulus dilatation may affect the feasibility and choice of surgical/percutaneous repair techniques (4). Chronic severe primary MR leads to LV volume overload, which is accompanied by a progressive LV and left atrial remodelling, gradual rise in pulmonary arterial pressure, and a significant right atrial annular dilatation (5,6).
Treatment decisions in primary MR are typically based upon symptomatic status, and resting imaging determination of its aetiology, severity, and consequences (1,2). Surgery is recommended in patients who are symptomatic at rest or during exercise testing (class IB). In asymptomatic patients, pre-operative resting LV ejection fraction <60% (ESC IC, AHA/ACC IB), end-systolic diameter >40 mm (AHA/ACC IIaB) >45 mm (ESC IIaC), recurrent atrial fibrillation, or pulmonary artery systolic pressure (PASP) >50 mmHg (ESC IIaC, AHA/ACC IIaB) represent common indications for mitral valve surgery, preferably repair. Surgery may be considered in asymptomatic patients with preserved LV function, high likelihood of durable repair, low surgical risk, and pulmonary hypertension on exercise (SPAP ≥60 mmHg at exercise) (ESC IIbC).
The development of surgical mitral valve repair, introduced in the early seventies by Alain Carpentier, has dramatically changed the prognosis and management of patients presenting with severe primary MR (7). Reconstructive surgery has progressively become the reference in the treatment of degenerative MR due to the low incidence of valve-related complications (i.e., endocarditis, thromboembolic/bleeding events) and the positive effect on life expectancy (8). However, the success of the intervention depends on additional factors, including surgeon’s skill, and severity of valve lesions. Indeed, recurrence of MR is far from exceptional; it is a recognized morbidity owning to both procedural failures and continuing degenerative process (9). Recurrent MR is reported as one of the most frequent conditions for mitral valve reoperation following repair. In most cases of reoperation (>50%), mitral valve replacement with a prosthetic valve needs to be performed due to advanced progression of the degenerative disease. According to published literature, the global percentage of patients undergoing mitral valve reoperation for any reason ranges from 0.7% to 10.5% (median 2.5%); however, the time before reoperation remains unclear. Freedom from mitral valve reoperation at 1-year and 5-year is typically reported as >94% (9).
A wide variety of surgical approaches for mitral valve repair are available, which aim at restoring natural leaflet motion, while preserving a large valve opening with leaflets that fit well together (10). Recent studies have demonstrated that the techniques which “respect (anatomical re-arrangement of tissues) rather than resect” the diseased portion of the mitral valve have comparable clinical outcomes, and are potentially more efficient to maintain mitral valve dynamics (11). During the last few decades, replacement of chords with expanded polytetrafluoroethylene (ePTFE) sutures has become a standard tool in reconstructive mitral surgery, both by standard and minimally invasive approaches (12). Indeed, this strategy is preferred to chordal shortening or chordal transfer, which has not always led to predictable and durable results (13,14). Artificial chordae couple the mitral valve and LV, and restore anatomical mitral valve structure and function by retaining the tension between the valve leaflet and the subvalvular apparatus. Replacement of chords is a safe and effective means of correcting anterior or posterior mitral valve prolapse with good long-term results. The extensive use of artificial chordae in cases of complex lesions involving the anterior or the two leaflets allows mitral valve repair in more than 95% of cases (15). Moreover, this approach could limit or avoid leaflet resection in extensive posterior mitral valve prolapse, which could be unamenable to conventional resecting repair strategies. In a recent randomized controlled trial, the use of loop neo-chords for correcting posterior mitral valve prolapse resulted in similar clinical and echocardiographic outcomes, but greater line of coaptation, as compared to conventional leaflet resection techniques (16). Until recently, replacement of chords has required open-heart surgery with bypass to stop the heart during the procedure (17).
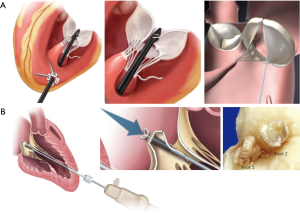
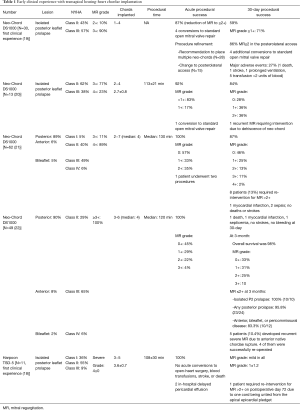
Transapical beating-heart mitral valve repair has been freshly introduced to treat MR while potentially reducing surgical morbidity. The NeoChord DS1000 has been the first device to be clinically approved for mitral valve repair by implantation of neo-chordae in patients presenting with degenerative MR (prolapse or flail). The system is introduced through the lowest part of the beating heart (lateral to apex), into the LV, and between the mitral valve leaflets. The prolapsed leaflet is then grasped, and, when adequately captured, the ePTFE suture is deployed and attached to the leaflet (Figure 1). The suture is then pulled through the apex of the heart as the DS1000 is removed. The correct length of the suture is determined by observing the improvement in MR in the beating heart. Early results from the Transapical Artificial Chordae Tendinae (TACT) trial demonstrated acute procedural success in 26 of the 30 patients, with only 17 patients with <2+ grade MR at 30 days (19) (Table 1). About 20% of the patients underwent reoperation for a failed repair and 16.7% of the patients required greater than 2 units of blood transfusion for blood loss at the catheter entry site in the ventricular apex. During the course of the study, 2 procedural refinements were introduced, including the revision of the LV access to postero-lateral approach (reduction of mechanical stress due to the posterolateral fixation of neo-chordae) and the use of multiple neo-chordae per procedure (better distribution of mechanical stress on leaflet tissue) (19). Subsequent studies showed high feasibility and confirmed good safety. The procedure success was related to mitral valve anatomy, and was less evident in anterior, bileaflet, or peri-commissural disease. A recent post-market surveillance registry conducted at 7 European centres has completed the enrolment of 126 patients with severe degenerative MR. Data from 2 of these centres confirmed initial results and persistent short-term efficacy with significant clinical benefit for the patients (20-22).
Full table
Harpoon TSD-5 is in the same category as “NeoChord” and is designed to facilitate beating-heart minimally invasive echo-guided mitral valve repair. It is approved for degenerative MR. A key feature of the system is that it automates a critical part of the repair process, simplifying the procedure and reducing its duration. In this device, a pre-formed ePTFE knot is deployed on the atrial surface of the prolapsing flail mitral leaflet, away from the leaflet edge, and towards the belly (Figure 1). Compared with the Neochord device, the Harpoon device is characterized by a smaller-diameter shaft (3 vs. 8 mm), a valved introducer to minimize intraprocedural bleeding, the ability to insert the ePTFE cords anywhere on the leaflet rather than within 4 mm of the free edge, and a fundamentally different anchoring mechanism. Gammie et al. recently reported the results of the first safety study of the device. This study involved a total of 11 patients with severe degenerative MR resulting from isolated posterior leaflet prolapse who underwent the procedure at two facilities in Poland. The authors reported a 100% procedural success rate with stable results after 30 days. An average of 3.6±0.7 (range 3–5) pairs of ePTFE artificial cords were implanted, with reduction of MR during the procedure from severe to none/trace in 8 patients and to mild in 3 patients. Total procedure time averaged 108±30 minutes (range, 72–167 minutes). The procedure was safe with no acute conversions to open-heart surgery, blood transfusions, stroke, or death. MR reduction was stable in the 3 of 4 patients followed-up to 6 months (18). Of note, there was some progression of MR from no patients having moderate MR and 3 with mild MR at the end of the procedure to 2 moderate and 5 mild MR at 30 days.
The benefits of mitral valve repair over replacement are indisputable and the trend towards more repair techniques in MR is predicted to continue. The most straightforward application of transapical beating-heart artificial chordae implantation concerns the case of isolated prolapse of a single mitral valve leaflet due to degenerative disease with limited mitral annulus dilatation and LV remodelling. Often two or more chordae are necessary to get good MR reduction. These transapical approaches have some advantages because they simplify the procedure, they preserve the subvalvular apparatus, and they facilitate both implantation and tension adjustment (as the heart is fully loaded and beating) of the neo-chordae under transesophageal echocardiographic guidance. However, the disadvantage of the technique is that it creates neo-chordae with a fixed length, which relies solely on echocardiographic evaluation. Indeed, minor errors in chordae length can have dramatic consequences on the tension of the chordae (23). The chordae are more than simple connections between the papillary muscles and the mitral annulus. Variations in the length of individual chordae modulate overall chordae tension, the coaptation area of the mitral leaflets and mitral hemodynamics especially in the absence of remodelling annuloplasty whose role in evenly distributing constraints along the mitral valve complex is well documented (24). Chordae that are longer than normal do not make the mitral valve incompetent, but can increase adjacent chordae tension, while using shorter-than-normal chordae can produce stress concentrations at both ends of the neo-chordae. At the free edge of the leaflet this can impair proper mitral valve closure or lead to dehiscence at the anchoring site. Increased tensile stress on the PTFE chords can also possibly facilitate subsequent rupture (25). Mechanical stress in traction close to the base of the papillary muscle can trigger by different mechanisms arrhythmias as observed in some forms of mitral valve prolapse (5). These reasonable Speculations/hypotheses emphasize the “a priori” critical impact of length adjustment and related forces applied to the mitral valve apparatus on the outcome. A potential drawback of the Neo-Chord DS1000 is its limited ability to restore coaptation along the entire flailing/prolapsing edge. Conversely, the Harpoon TSD-5, by supporting the leaflet belly, may be sufficient to parallelize the leaflet with the non-prolapsing leaflet, and achieve leaflet coaptation. However, this is purely hypothetical and whether the system can realize a better positioning remains unclear. Nevertheless, it is likely that a positioning further away from the edge of the leaflet should reduce the amount of tissue available for coaptation. The pre-formed knot may potentially overcome the shortcomings of focal NeoChord loop deployment (zone of focal stress), and supports a larger region (no inter-loop persisting prolapse) of the leaflet with a single neo-chord. A key advantage of the Neo-Chord remains, however, the possibility to be removed prior to the creation of the knot, while the Harpoon’s cords cannot be removed once fired.
Transcatheter mitral valve repair technologies have progressively gained widespread interest, and have widely been adopted. Transapical beating-heart artificial chordae implantation represents an additional approach to the current options for mitral valve repair, and will likely become an important component of the mitral valve surgeon’s armamentarium. Conversely, transfemoral approach (i.e., MitraClip) will certainly remain the preferred option of interventional cardiologists. With the possibility of using the Neo-Chord DS1000 or the Harpoon TSD-5 in both high and low risk patients, the level of repair perfection remains very high given the excellent results of conventional surgery. However, while promising, the routine use of such novel technology requires additional experience and a better understanding of the following aspects: learning curve, exact positioning of the neo-chordae to the prolapsing segment; length adjustment of the neo-chord to eliminate prolapse; prevention of leaflet restriction; impact of apical fixations on mitral valve structure and function (higher rate of change of force in the chord as compared to papillary muscles insertion); possible injury of mitral valve leaflets/chordae tendineae; risk of MR progression/recurrence without concomitant annuloplasty, interest of combined transcatheter techniques (i.e., the Valtech trans-septal annuloplasty band), long-term results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007;28:230-68. [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438-88. [Crossref] [PubMed]
- Lancellotti P, Tribouilloy C, Hagendorff A, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611-44. [Crossref] [PubMed]
- Stone GW, Vahanian AS, Adams DH, et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 1: clinical trial design principles: A consensus document from the mitral valve academic research consortium. Eur Heart J 2015;36:1851-77. [Crossref] [PubMed]
- Lancellotti P, Garbi M. Malignant Mitral Valve Prolapse: Substrates to Ventricular Remodeling and Arrhythmias. Circ Cardiovasc Imaging 2016;9:e005248. [Crossref] [PubMed]
- Lancellotti P, Martinez C, Bernard A. Pulmonary Pressures and Outcome in Primary Mitral Regurgitation: Paradigm Shift From Rung to Ladder. J Am Coll Cardiol 2016;67:2962-4. [Crossref] [PubMed]
- Carpentier A. Cardiac valve surgery--the "French correction". J Thorac Cardiovasc Surg 1983;86:323-37. [PubMed]
- Mohty D, Orszulak TA, Schaff HV, et al. Very long-term survival and durability of mitral valve repair for mitral valve prolapse. Circulation 2001;104:I1-I7. [Crossref] [PubMed]
- Jouan J, Berrebi A, Chauvaud S, et al. Mitral valve reconstruction in Barlow disease: long-term echographic results and implications for surgical management. J Thorac Cardiovasc Surg 2012;143:S17-20. [Crossref] [PubMed]
- Glower DD. Surgical approaches to mitral regurgitation. J Am Coll Cardiol 2012;60:1315-22. [Crossref] [PubMed]
- Bellitti R, Petrone G, Buonocore M, et al. Anatomic reconstruction in degenerative mitral valve bileaflet prolapse: long-term results. Ann Thorac Surg 2014;97:563-8. [Crossref] [PubMed]
- David TE, Armstrong S, Ivanov J. Chordal replacement with polytetrafluoroethylene sutures for mitral valve repair: a 25-year experience. J Thorac Cardiovasc Surg 2013;145:1563-9. [Crossref] [PubMed]
- Phillips MR, Daly RC, Schaff HV, et al. Repair of anterior leaflet mitral valve prolapse: chordal replacement versus chordal shortening. Ann Thorac Surg 2000;69:25-9. [Crossref] [PubMed]
- Sousa Uva M, Grare P, Jebara V, et al. Transposition of chordae in mitral valve repair. Mid-term results. Circulation 1993;88:II35-8. [PubMed]
- Castillo JG, Anyanwu AC, Fuster V, et al. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: implications for future guidelines. J Thorac Cardiovasc Surg 2012;144:308-12. [Crossref] [PubMed]
- Falk V, Seeburger J, Czesla M, et al. How does the use of polytetrafluoroethylene neochordae for posterior mitral valve prolapse (loop technique) compare with leaflet resection? A prospective randomized trial. J Thorac Cardiovasc Surg 2008;136:1205; discussion 1205-6. [Crossref] [PubMed]
- Ibrahim M, Rao C, Athanasiou T. Artificial chordae for degenerative mitral valve disease: critical analysis of current techniques. Interact Cardiovasc Thorac Surg 2012;15:1019-32. [Crossref] [PubMed]
- Gammie JS, Wilson P, Bartus K, et al. Transapical Beating-Heart Mitral Valve Repair With an Expanded Polytetrafluoroethylene Cordal Implantation Device: Initial Clinical Experience. Circulation 2016;134:189-97. [Crossref] [PubMed]
- Seeburger J, Rinaldi M, Nielsen SL, et al. Off-pump transapical implantation of artificial neo-chordae to correct mitral regurgitation: the TACT Trial (Transapical Artificial Chordae Tendinae) proof of concept. J Am Coll Cardiol 2014;63:914-9. [Crossref] [PubMed]
- Rucinskas K, Janusauskas V, Zakarkaite D, et al. Off-pump transapical implantation of artificial chordae to correct mitral regurgitation: early results of a single-center experience. J Thorac Cardiovasc Surg 2014;147:95-9. [Crossref] [PubMed]
- Colli A, Bellu R, Pittarello D, et al. Transapical off-pump Neochord implantation on bileaflet prolapse to treat severe mitral regurgitation. Interact Cardiovasc Thorac Surg 2015;21:554-6. [Crossref] [PubMed]
- Colli A, Manzan E, Zucchetta F, et al. Transapical off-pump mitral valve repair with Neochord implantation: Early clinical results. Int J Cardiol 2016;204:23-8. [Crossref] [PubMed]
- Reimink MS, Kunzelman KS, Cochran RP. The effect of chordal replacement suture length on function and stresses in repaired mitral valves: a finite element study. J Heart Valve Dis 1996;5:365-75. [PubMed]
- Stevanella M, Votta E, Redaelli A. Mitral valve finite element modeling: implications of tissues' nonlinear response and annular motion. J Biomech Eng 2009;131:121010. [Crossref] [PubMed]
- Butany J, Collins MJ, David TE. Ruptured synthetic expanded polytetrafluoroethylene chordae tendinae. Cardiovasc Pathol 2004;13:182-4. [Crossref] [PubMed]


