The impact of operative approaches on outcomes of middle and lower third esophageal squamous cell carcinoma
Introduction
It is estimated that 455,800 new esophageal cancer cases and 400,200 deaths occurred in 2012 worldwide (1). According to the annual report of National Cancer Center of China, 291,238 new esophageal cancer cases and 218,957 deaths occurred in China during 2011 (2). Therefore, esophageal cancer is a detrimental disease to worldwide, especially to China.
Currently, surgery remains the mainstay for resectable esophageal cancer (3). McKeown approach is the main approach for upper and middle third esophageal cancer (4). While there were two main approaches for middle and lower third esophageal cancer: Ivor Lewis and Sweet approaches. An international survey on esophageal cancer showed that Ivor Lewis was the most common approach used in western countries (5). Ivor Lewis approach is more convenient in improving visualization of mediastinal structures, decreasing frequency of recurrent laryngeal nerve injuries. Moreover, it is reported that a comprehensive thoracic lymph node harvest and the creation of a tension-free anastomosis between the remnant oesophagus and the gastric conduit are achieved easier with Ivor Lewis (6). However, in Asia, especially in China, the Sweet was the most common approach and it had many advantages including adequate exposure of the stomach and excellent access to the short and left gastric arteries through the opening in the left hemidiaphragm (7). In addition, rapid and simple wound opening and closing, reduced operative times in Sweet approach, implicate for the value of its use in the surgical treatment of middle or lower third esophageal cancer. Therefore, both the Sweet and the Ivor Lewis approaches may be suitable for surgical treatment of middle or lower third esophageal cancer (8).
There are conflicting results regarding the effectiveness of Sweet versus Ivor Lewis approach (9-11). Fu et al. reported that it is easier to perform systemic lymphadenectomy via right thoracic approach than left approach and the local recurrence is reduced and long-term survival improved (9). On the contrary, Ma J and Ma Q reported that Sweet approach was associated with reduced duration of operation, decreased complication rate, similar long term survival in esophageal cancer patients (7,10). A meta-analysis including 15 studies demonstrated that two approaches had similar long term effect (8). A recent randomized controlled trial comparing left and right approaches concluded that Ivor-Lewis and Sweet esophagectomies are both safe procedures with low operative mortalities (11). However, no long-term survival was reported in that study.
The aim of this study was to investigate the short-term outcomes and long term survival comparing Sweet and open Ivor Lewis esophagectomies in the surgical treatment of middle and lower third esophageal cancer using propensity score matching analysis method in a high-volume cancer center.
Methods
This study was a single-center retrospective study and was approved by the Institutional Review Board of Cancer Hospital (ethics number NCC2013SF-10), Chinese Academy of Medical Science and Peking Union Medical College. The medical records of 1,746 consecutive patients who underwent open esophagectomy for middle and lower esophageal cancer between January 2009 and September 2015 in the First Department of Thoracic Oncologic Surgery of Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College were retrospectively reviewed. The clinical variables included age, sex, age-adjusted Charlson score, use of neoadjuvant therapy, tumor location, duration of surgery, estimated intraoperative blood loss, number of harvested lymph nodes, postoperative morbidity rate, mortality rate, hospital length of stay (LOS), and 3-year survival were compared between Sweet and open Ivor Lewis approaches. All patients were diagnosed as squamous cell carcinoma in postoperative pathology. Hospital expense was also recorded.
Age-adjusted Charlson score was carried out according to the definition of Koppie et al. (12). Esophageal cancer staging was assessed according to American Joint Committee on Cancer (AJCC) 2010 cancer staging (13). Postoperative complications were recorded based on international consensus on standardization of data collection for complications associated with esophagectomy (14). There were nine categories of complications including pulmonary, cardiac, gastrointestinal, urologic, thromboembolic, neurologic/psychiatric, infection, wound/diaphragm and other. The major and minor complication data were scored with Clavien-Dindo classification (15).
Surgical technique
The choice of surgical approach for middle and lower third esophageal cancer was mainly based on the preference of surgeons. Before 2009, Sweet approach was the predominant approach. And since 2009, open Ivor Lewis esophagectomy were undertaken by some surgeons to dissect malignant lesions of middle and lower third esophagus. The details of three approaches have been elaborated in our previous studies (16,17).
Statistics
The SPSS software package 16.0 for Windows was used for statistical analysis. Data were presented as mean value ± standard deviation for continuous variables, and percentages for dichotomous variables. Continuous variables were analyzed using t-test or nonparametric test, and categorical variables were analyzed using Fisher test. Survivals were estimated using Kaplan-Meier methods and log-rank tests were used to analyze differences between curves. We made propensity score matching analysis according to Austin PC (18). For propensity score matching analysis, we first made the logistic regression model that calculated propensity scores matching using approach (Sweet or Ivor Lewis approach) as outcome with age, sex, BMI, age-adjusted Charlson score, tumor location, AJCC staging and neoadjuvant therapy and/or chemotherapy. The significant level was set as a P value less than 0.05.
Results
Demographics
A total of 1,746 consecutive patients with middle and lower esophageal squamous cell carcinoma underwent esophagectomy from January 2009 to September 2015 at the First Department of Thoracic Oncologic Surgery of Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Of these 1746 patients, 1,701 patients received esophagectomy by Sweet approach, 45 patients by open Ivor Lewis approach.
In this cohort, the mean age was 59.9 years in the Sweet group, which is higher than 58.9 years in the open Ivor Lewis group. More patients in the Sweet group had middle esophageal cancer than patients in the open Ivor Lewis group (97.1% vs. 77.8%; P<0.001). There were no significant differences in age-adjusted Charlson score, body mass index, and rate of receiving neoadjuvant therapy between Sweet and open Ivor Lewis groups (Table 1).
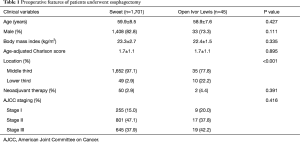
Full table
Surgical outcomes
As shown in Table 2, patients who received esophagectomy by Sweet approach had shorter duration of surgery compared with patients received open Ivor Lewis approach (mean 212 vs. 390 min; P<0.001). Patients who received esophagectomy by Sweet approach had more lymph nodes compared with patients by open Ivor Lewis approach (mean 24 vs. 19; P=0.005). And overall complications rate was higher in Ivor Lewis group than in Sweet group (24.4% vs. 11.7%; P=0.009). There was no significant difference in estimated intraoperative blood loss between Sweet and open Ivor Lewis approach (mean 241 vs. 239 mL; P=0.903).
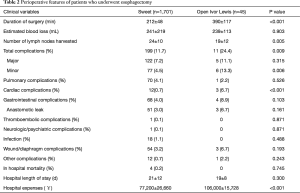
Full table
In order to balance preoperative variables, we made propensity score matching analysis. Preoperative variables of two groups are displayed in Table 3. After matching, Sweet approach was associated with decreased duration of surgery (mean 210 vs. 390 min; P<0.001), and more lymph nodes (mean 24 vs. 19; P=0.050) compared with Ivor Lewis approach (Table 4). There were no significant differences in overall complication rates (24.4% vs. 24.4%; P=1.000) between two approaches.
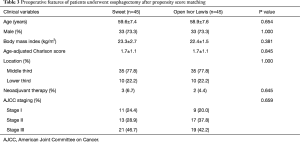
Full table
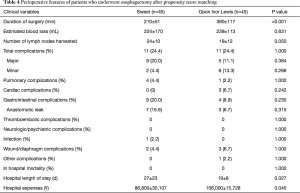
Full table
Hospital expense
The mean cost of Sweet group was ¥77,200, which was significantly lower than ¥106,000 in open Ivor Lewis group (P<0.001) in unmatched analysis, and total hospital in Sweet and Ivor Lewis approach was ¥86,800 and ¥106,000 (P=0.045) respectively in matched analysis.
Long term survival
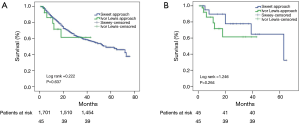
All patients were followed for 1 to 73 months (mean 12.64 months). There was no significant difference in 3-year overall survival (OS) between Sweet and open Ivor Lewis approaches (59.9% vs. 61.4%; P=0.637) in unmatched analysis as shown in Figure 1. After matching, the 3-year OS in Sweet and Ivor Lewis approach was 77.8% and 61.4% respectively (P=0.264).
Discussion
In this study, we found that there was no significant difference in short term outcomes and 3-year OS of cancer patients who received esophagectomy between Sweet and open Ivor Lewis approaches. However, the Sweet approach was associated with shorter duration of operation and decreased hospital expenses compared with open Ivor Lewis approach.
Overall complication rate was higher in open Ivor Lewis approach than in Sweet approach. However, only minor complication was significantly higher in Ivor Lewis group, and there was no significant difference in the major morbidity rate between these two approaches. A recent meta-analysis also demonstrated there were no significant differences in major morbidities including pulmonary complications and anastomotic leaks (8).
The number of lymph nodes harvested was higher in Sweet group than in Ivor Lewis group whether in unmatched and matched analysis. Learning curve may partly explain the results. In our previous study, at least 12 cases are needed to master minimally invasive McKeown esophagectomy (4). In this study, we attempted open Ivor Lewis since January 2009. However, oncologic result was not compromised in open Ivor Lewis approach, as there was no significant difference in 3-year OS between 2 approaches. Greenstein et al. demonstrated that patients who underwent esophagectomy should at least have 18 lymph nodes removed (19). The mean number of lymph nodes dissected in Sweet and Ivor Lewis was 24 and 19 respectively in our study, which was higher than the standard proposed by Greenstein.
Duration of surgery of patients who received esophagectomy by Sweet approach in our study was 212 min, which was significantly shorter than 390 min in open Ivor Lewis approach. This result is consistent with the results of Ma et al. and Li et al. (7,11). This phenomenon is easily explained by more number of Sweet approaches than Ivor Lewis approach in China.
Cost of Sweet approach was lower than that in open Ivor Lewis approach, which implies that for esophageal cancer patients in China, a developing country with low level medical coverage, Sweet approach maybe the first choice for the surgical treatment of middle and lower third esophageal cancer in most medical centers. However, in some large medical center with advanced medical technique, minimal invasive Ivor Lewis approach gained more popularity owning to lessened postoperative inflammatory reaction despite greater cost (20,21).
There was no difference in 3-year OS between 2 approaches in our study. Ma et al. reported that there was no significant difference between Sweet and Ivor Lewis approaches in terms of overall 5-year survival in nonrandomized controlled study (6). Ma et al. demonstrated that operation approach (left or right approach) was not risk factor for long term survival by Cox regression analysis in patients after esophagectomy, although univariable analysis showed that operation approach was an unfavorable prognostic factor for long term survival. While other factors such as tumor staging and age were unfavorable prognostic factors for long term survival in patients after esophagectomy (10). Recently, Li et al. conducted a randomized controlled study comparing the difference in postoperative complications between Sweet and Ivor approached and concluded that both approaches are safe procedures with low mortalities. However, no long-term survival was followed in their study (11). Therefore, more well-designed prospective studies are needed to clarify the role of operation approach in the long-term survival of patients who underwent esophagectomy.
There are some limitations in our study. Firstly, retrospective nature of this study, and relative small number of patients in open Ivor Lewis group in this study may preclude the generalization of the results of this study. However, we make propensity score matching analysis which comparing Sweet and Ivor Lewis approaches, which overcome the limitations of retrospective study. Secondly, the results of this study were achieved from data of esophageal squamous cell carcinoma and no esophageal adenocarcinoma was included, which imply that the conclusion of this study may not applicable to other countries or regions where esophageal adenocarcinomas are prevalent. Thirdly, neoadjuvant therapy is now the trend in combination therapy of squamous cell cancer, and the number of patients who received neoadjuvant therapy was relatively small. However, according to the recent guidelines of esophageal cancer (22), that randomized trials comparing surgery alone with preoperative chemoradiation followed by surgery in patients with clinically resectable cancer have shown conflicting results. Therefore, more studies are needed to assess the outcomes comparing surgery alone versus neoadjuvant therapy plus surgery. Lastly, Ivor Lewis approach was used only ten cases per year in our study, and the learning curve may weaken the results of this study. Therefore, well designed prospective studies are needed to clarify the effectiveness of Ivor Lewis vs. Sweet approach for the surgical treatment of middle and lower esophageal cancer.
Conclusions
In this cohort, for middle and lower third esophageal squamous cell carcinoma patients, both Sweet and open Ivor Lewis approaches are feasible in terms of perioperative outcomes and 3-year OS. The Sweet approach was associated with decreased hospital expenses and shorter duration of operation compared with open Ivor Lewis approach, which imply that the Sweet approach may be the first choice in developing countries from a cost-effective point of view.
Acknowledgements
Funding: This work was supported by the funding of Capital Health Technology Development Priorities Research Project (No. 2014-1-4021).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board at Cancer Hospital of Chinese Academy of Medical Sciences and Peking Union Medical College, and all patients provided written informed consent before operation (NCC2013SF-10).
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res 2015;27:2-12. [Crossref] [PubMed]
- Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499-509. [Crossref] [PubMed]
- Mu JW, Gao SG, Xue Q, et al. Updated experiences with minimally invasive McKeown esophagectomy for esophageal cancer. World J Gastroenterol 2015;21:12873-81. [Crossref] [PubMed]
- Boone J, Livestro DP, Elias SG, et al. International survey on esophageal cancer: part I surgical techniques. Dis Esophagus 2009;22:195-202. [Crossref] [PubMed]
- Sihag S, Wright CD, Wain JC, et al. Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis oesophagectomy at a single, high-volume centre. Eur J Cardiothorac Surg 2012;42:430-7. [Crossref] [PubMed]
- Ma J, Zhan C, Wang L, et al. The sweet approach is still worthwhile in modern esophagectomy. Ann Thorac Surg 2014;97:1728-33. [Crossref] [PubMed]
- Zhang H, Wang J, Wang W, et al. A meta-analysis of esophagectomy: the comparative study of Ivor-Lewis operation and Sweet operation. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:892-7. [PubMed]
- Fu SJ, Fang WT, Mao T, et al. Comparison of surgical outcomes after different surgical approach for middle or lower thoracic esophageal squamous cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2012;15:373-6. [PubMed]
- Ma Q, Liu W, Long H, et al. Right versus left transthoracic approach for lymph node-negative esophageal squamous cell carcinoma. J Cardiothorac Surg 2015;10:123. [Crossref] [PubMed]
- Li B, Xiang J, Zhang Y, et al. Comparison of Ivor-Lewis vs Sweet esophagectomy for esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg 2015;150:292-8. [Crossref] [PubMed]
- Koppie TM, Serio AM, Vickers AJ, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer 2008;112:2384-92. [Crossref] [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Zhang DW, Cheng GY, Huang GJ, et al. Operable squamous esophageal cancer: current results from the East. World J Surg 1994;18:347-54. [Crossref] [PubMed]
- Mu J, Yuan Z, Zhang B, et al. Comparative study of minimally invasive versus open esophagectomy for esophageal cancer in a single cancer center. Chin Med J (Engl) 2014;127:747-52. [PubMed]
- Austin PC. Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: a systematic review and suggestions for improvement. J Thorac Cardiovasc Surg 2007;134:1128-35. [Crossref] [PubMed]
- Greenstein AJ, Litle VR, Swanson SJ, et al. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer 2008;112:1239-46. [Crossref] [PubMed]
- Chen B, Zhang B, Zhu C, et al. Modified McKeown minimally invasive esophagectomy for esophageal cancer: a 5-year retrospective study of 142 patients in a single institution. PLoS One 2013;8:e82428. [Crossref] [PubMed]
- Mu J, Gao S, Mao Y, et al. Open three-stage transthoracic oesophagectomy versus minimally invasive thoraco-laparoscopic oesophagectomy for oesophageal cancer: protocol for a multicentre prospective, open and parallel, randomised controlled trial. BMJ Open 2015;5:e008328. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. [PubMed]


