Relationship between radiologic patterns, pulmonary function values and bronchoalveolar lavage fluid cells in newly diagnosed sarcoidosis
Introduction
Sarcoidosis is a systemic granulomatous disease of unknown aetiology that primarily affects the lungs, although multiorgan involvement frequently occurs (1). Imaging makes an important contribution to the assessment of prognosis and follow-up in sarcoidosis (2). Pulmonary sarcoidosis may manifest with various radiologic patterns. There are five radiologic stages (forms) of intrathoracic sarcoidosis: stage 0, normal chest radiograph; stage I, mediastinal lymphadenopathy only; stage II, mediastinal lymphadenopathy with parenchymal lesion; stage III, parenchymal disease only; and stage IV, pulmonary fibrosis (3,4).
Radiological staging systems have been in use for many years and are still of value in pulmonary sarcoidosis. Moreover, these systems may be somewhat useful in predicting the likelihood of disease remission (1). For the most part, the prognosis is based upon the findings of chest radiography. Although the condition either regresses or remains stable in most patients, it can progress to pulmonary fibrosis in approximately one-quarter of patients. Spontaneous resolution occurs in 60–90% of patients with stage I disease, and 40–70% of those with stage II (5). That is to say, the frequency of variations in the course of sarcoidosis in stages I, II and III are very high and additional prognostic indictors are needed. Radiographic staging was developed before the introduction of computed tomography (CT). CT, especially high-resolution CT (HRCT), is far more sensitive than chest radiography in discerning subtle parenchymal abnormalities in the early stages of the disease (6). Chest CT allows for the identification of pathological changes not seen in conventional chest radiographs. CT, especially HRCT, may be particularly useful for distinguishing active inflammation from irreversible fibrosis in patients with stage II or stage III sarcoidosis (6).
Though patients with the acute form of the disease (i.e., Löfgren’s syndrome) have a favourable prognosis, the course of sarcoidosis cannot currently be predicted using any biological markers. Moreover, there is a practical issue as it remains unclear which diagnostic test or biological marker may be the most suitable for follow-up examination. Although recently several publications (7-9) demonstrated potential benefits of positron emission tomography (PET) in evaluating the extent of sarcoidosis and the disease inflammatory activity, it is unlikely that PET will be useful in daily clinical practice considering the cost and little additional benefit in patient management (10). Future larger scale research studies are needed to further elucidate the role of PET in the management of patients with sarcoidosis (11).
Consequently, no evidence based guidelines are available to indicate how often sarcoid patients should be followed-up after initial diagnosis (12,13). With the role of chest CT in sarcoidosis still not fully defined and requiring further study (2), it is necessary to investigate the relationship between CT findings and other markers.
Recently, the correlation between disease severity in CT, functional impairment and radiographic stages of sarcoidosis, as well as pulmonary function tests (PFT) and bronchoalveolar lavage fluid (BALF) cells, have been discussed in the literature (5,14-27). However, few systematic evaluations exist exploring the relationships between the various HRCT findings (e.g., micronodules, macronodules, irregular linear opacities, interlobular septal thickening, fibrotic changes, etc.), respiratory function and the course of sarcoidosis.
The primary aim of the present study was to identify specious radiologic and/or physiologic prognostic marker(s), which lead to optimize of the patient follow-up frequency. In this paper, we present the first part of our results: the relationships between various lung changes on CT scans, PFT and BALF cells.
Methods
The study sample consisted of 80 consecutive patients newly diagnosed with pulmonary sarcoidosis between 2011 and 2012 at Center of Pulmonology and Allergology, Vilnius University Hospital (Santariskiu Klinikos). Patients underwent diagnostic testing as a part of routine clinical investigation. None of the subjects had any relevant medical history or comorbidity (e.g., tuberculosis). None of patients had a history of home or workplace exposure to organic or mineral dusts known to cause granulomatous lung disease.
The patients were 41 (51%) females and 39 (49%) males with a mean age of 39±9 years. All of the patients were the Lithuanian descent, Caucasians. We did not have familial forms of sarcoidosis. 16 patients (20%) were smokers. There were no patients with stages 0 and IV sarcoidosis during the enrolment period. The study population demographic data, sorted according to the chest radiographic stages of sarcoidosis, is summarized in Table 1.
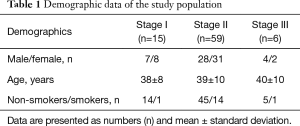
Full table
The patients underwent chest radiography, HRCT examination, PFT [including diffusing capacity of carbon monoxide (DLCO)], fiberoptic bronchoscopy with BAL and bronchoscopic lung biopsy, and BALF cell examination. All study tests were performed within 2 weeks or less than 1 month from the first manifestation of the disease. The diagnosis was confirmed according to ATS/ERS/WASOG statement (1). A signed informed consent form was obtained from each participant. The study was approved by the Vilnius Regional Biomedical Ethics Committee (No. 158200-12-559-160).
CT scanning
HRCT scans were performed using 64-slice CT (GE LightSpeed VCT, GE Healthcare, Milwaukee, Wisconsin, USA). Scan parameters were as follows: collimation of 64 mm × 1.25 mm, tube voltage 120 kV, tube current 660 mAs.
The following patterns were evaluated: micronodule (2–7 mm in diameter), macronodule (8–30 mm in diameter), consolidation, ground glass opacity and linear opacities (28). The distribution and extent of the parameters on thorax CT scans were determined: parenchymal areas above the main carina were upper zones, areas below lower pulmonary veins were lower zones and areas between two zones were the middle zones of each lung. A profusion score for each morphologic abnormality was assigned to each lung zone based on the percentage of the lung zone involved: 0 point (no involvement), 1 point (1–25%), 2 points (26–50%), 3 points (51–75%) and 4 points (>75% of lung zone). A nodule profusion score was based on the number of nodules per zone: 0= no nodules, 1=1–5 nodules, 2=6–10 nodules, 3=11–15 nodules, and 4=>15. The sum of the scores and the mean values of each parameter for each patient were defined. For each parameter, a patient might have a maximum of 24 points (15).
The presence of typical [i.e., hilar, mediastinal (right paratracheal), bilateral, symmetric, ≥15 mm in diameter] and atypical (i.e., unilateral, isolated, anterior and posterior mediastinal) localization of lymph nodes and the occurrence of lymph node calcification were determined (6).
PFT
Pulmonary function testing included the measurement of forced vital capacity (FVC), forced expiratory volume in one second (FEV1), a determination of total lung capacity (TLC), vital capacity (VC), residual volume (RV) with body plethysmography, and DLCO with standard single-breath technique Vmax Encore (Viasys® Healthcare, USA). Results were expressed as percentages of predicted normal values.
Bronchoscopy and bronchoalveolar lavage
Fiberoptic bronchoscopy and bronchoalveolar lavage were performed as described elsewhere (18,29,30). BALF analysis was performed as described in previous publications (17,18). We used the same BAL and BALF methods of analysis throughout the study period.
Statistical analysis
Group data was expressed as mean values ± standard deviation (SD). Pearson’s and Spearman’s correlation coefficients were used to investigate the correlation between the results of pulmonary function measurements and BALF cells. Data was compared using a Kruskal-Wallis test with Dunn’s post hoc test for multiple comparisons. Data of different groups (e.g., smoker and non-smoker patients) were compared using the Mann-Whitney U-test. In all tests, a P value of <0.05 was considered to be statistically significant. Statistical data was processed using SPSS, version 17.0 (Statistical Package for Social Sciences, IBM, USA).
Results
CT findings
Sixty-four patients (80%) had typical lymphadenopathies, whereas 36 patients (20%) had an atypical localization of lymph nodes. There were 14 patients (17.5%) with calcinosis of lymph nodes.
Micronodules (77.5%), macronodules (53.8%) and linear opacities (45%) were the most frequently seen CT patterns in patients with pulmonary sarcoidosis. Notwithstanding, the number of micronodules was significantly higher (P=0.004) in stage III as compared with the other stages (Table 2). We found that micronodules and macronodules were, for the most part, symmetrical located in the middle zones of the lungs; linear opacities, located in the upper and middle zones of the lungs; and ground glass opacities, located in the lower zones of the lungs.
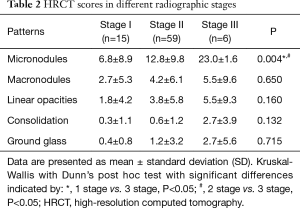
Full table
Pulmonary function parameters
Sixteen patients (20%) had a restrictive pattern (TLC <80). Forty four patients (55%) had an altered DLCO (<80).
We found statistically significant differences in several pulmonary function parameters among the patient groups with different radiographic stages of sarcoidosis. The patients at radiographic stage I had better PFT as compared to those in stages II and III, respectively. A decrease of the DLCO was the most commonly observed impairment of the lung function in our patients. The results of the PFT are summarized in Table 3.
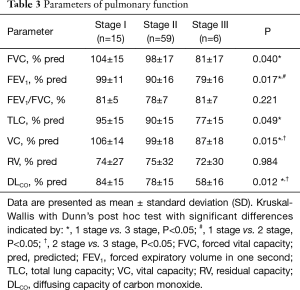
Full table
Although we observed a decrease in all pulmonary function parameters (FVC, FEV1, FEV1/FVC, TLC, VC and DLCO) among smokers as compared with non-smokers, the differences were not statistically significant.
Significant correlations between the consolidation scores on the CT and FVC (r=−0.227, P=0.043), FEV1 (r=−0.299, P=0.007), FEV1/FVC (r=−0.245, P=0.029), as well as between the ground glass opacity score and DLCO (r=−0.267, P=0.017), were established. Surprisingly, we did not find any significant correlations between the micronodule or macronodule scores and PFT indices. However, the presence of calcinosis in lymph nodes correlated negatively with FEV1 (r=−0.44, P=0.008).
BALF cells differentials
There were significant differences (P=0.021) between the percentage of BALF neutrophils in radiographic stages I and III (Table 4). We observed a significant increase in the proportion of CD8+ T lymphocytes (P=0.005), as well as a decrease in the proportion of both CD4+ T lymphocytes (P=0.035) and the CD4/CD8 ratio (P=0.011) in smokers (40%, 53% and 2.8, accordingly) as compared with non-smokers (22%, 68% and 5.1, respectively) in stage II. There was only the one smoking patient in stage I and stage III; therefore, we did not compare smokers and non-smokers in these groups.
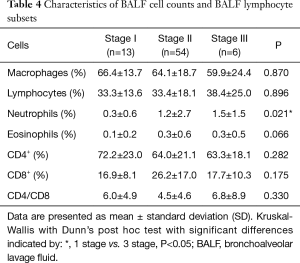
Full table
We found that the percentage of BALF lymphocytes in patients with restrictive pattern compared with no restrictive pattern was increased (44.6%±16.5% and 30.8%±17.0% respectively). The percentage of BALF lymphocytes and macrophages correlated with TLC values: the percentage of lymphocytes was negatively correlated (r=−0.27, P=0.02) and the percentage of macrophages was positively correlated (r=0.27, P=0.02). Furthermore, BALF cells correlated with the presence of typical lymphadenopathy: the percentage of neutrophils correlated negatively (r=−0.41, P=0.015), and the percentage of CD4+ cells and the CD4/CD8 ratio both correlated positively (r=0.38, P=0.026; r=0.3, P=0.078, respectively).
Discussion
The data presented reflects a cross-sectional analysis of radiography/PFTs/BALF cells in a cohort of sarcoidosis patients. The key findings of our study, with respect to identifying the best prognostic marker(s) for the prediction of disease progression and for determining the frequency of follow-up checks, are described herein.
Firstly, the reduction in PFT values seen in radiological sarcoidosis stage III (with the most sizeable decrease of the DLCO level) was greater than that seen in stages I and II. Secondly, as the disease advanced, the percentage of neutrophils in the lungs was found to increase, as compared with stage I. Thirdly, pulmonary function indices were correlated negatively with the consolidation and ground glass opacities CT scores, but not with the micronodule or macronodule scores. Fourthly, the rise in the percentage of BALF lymphocytes was associated with the restriction pattern of pulmonary function. Other important findings were that the diagnostic value of BALF for sarcoidosis [lymphocytosis and CD4/CD8 >3.5 (30)] was higher when the typical radiologic patterns of stage I disease were found and that smoking decreased the diagnostic value of CD4/CD8 ratio.
A characteristic feature of pulmonary sarcoidosis is an increase in CD4+ T cells and the CD4/CD8 ratio in the BAL fluid. However, cellular components of BALF and T lymphocyte profiles are associated with several situations, such as clinical presentation, radiological stage and smoking status (17,18). A high relative CD4+ T lymphocyte number (percentage) and high CD4/CD8 ratio, increased alveolar macrophages amount in BALF reflect an active inflammatory process in the lung. The increased neutrophil number, decreased lymphocyte count and CD4/CD8 ratio are associated with an increased radiographic stage of sarcoidosis. It is thought that the increased BALF neutrophil and/or eosinophil count is associated with a more advanced, chronic disease course (17,31-33). The increase of the macrophages and neutrophil count, decrease of lymphocyte count and CD4/CD8 ratio with increased radiographic stage of sarcoidosis in BALF in patient with newly diagnosed sarcoidosis have been documented (17,23). In line with these observations, we found significant differences (P=0.021) between the percentage of neutrophils of BALF in radiographic stages I and III in our sample. Other findings (CD4+ T lymphocyte, CD4/CD8 ratio) were not statistically significant, maybe due to the low number of patients in radiographic stage III. Additionally, it is likely that the relatively high proportion of smokers in stage II of our sample had an effect on the BALF cell count, especially on the CD4/CD8 ratio. However, we have observed a significant increase in the proportion of CD8+ cells (P=0.005), as well as decrease in both the proportion of CD4+ cells (P=0.035) and CD4/CD8 ratio (P=0.011) in smokers (40%, 53% and 2.8, accordingly) as compared with the non-smokers (22%, 68% and 5.1, respectively) in stage II. Our results support the view that cigarette smoking modifies the immunologic BALF sample profile and that alveolitis appears less pronounced in smokers. Usually, number of lymphocytes, CD4+ cells and CD4/CD8 ratio is lower in smokers than in non-smokers (34).
Respiratory function is closely related to the severity and extent of inflammatory changes in the alveolar walls. In the presence of alveolar wall inflammation, gas diffusion in the lungs is disturbed. One of the earliest and most common abnormalities of sarcoidosis is reduced DLCO. As the disease progresses, respiratory tract obstruction occurs (31-33). We determined statistically significant differences in the pulmonary function parameters of patients with different radiographic stages of sarcoidosis: PFT parameters significantly decreased from sarcoidosis stage I to stage III. A decrease of the DLCO was the most commonly observed impairment of the lung function in our patients. However, our data shows that, for most of I and II stage patients, the PFT values were normal, and the dysfunction was found while performing DLCO.
The other authors evaluated the role of serial chest X-ray and serial measurement of PFT in sarcoidosis (16). They found the discordance between pulmonary function data and chest X-ray data in 50% of cases and concluded that changes in radiographic extent are more applicable to routine monitoring in sarcoidosis than change in radiographic stages. PFT parameters decrease in higher radiographic stages (17,35,36). We found that the percentage of BALF lymphocytes and macrophages correlated with the TLC values: the percentage of lymphocytes correlated negatively and the percentage of macrophages correlated positively. This was an unexpected outcome, because the increased proportion of neutrophils in BALF is more common in the later radiographic stages of sarcoidosis, where fibrotic changes occur and lung capacities, as well as volumes, become lower.
Our study showed that the most common HRCT abnormality found in patients with pulmonary sarcoidosis were abnormalities in the pattern of micronodules. This finding was not surprising, because from a pathological point of view, sarcoidosis is characterized by the formation of small, granular inflammatory lesions (granulomas) and the perilymphatic interstitial distribution of granulomas is characteristic. Although sarcoid granulomas arise as micronodular lesions, they may coalesce over time, forming larger lesions (i.e., macronodules) (4,37) or confluences of several micronodules can form macronodules (28). However, it is not possible to accurately determine the age of a pulmonary injury or whether the changes are reversible, if they are only present on CT scans. We hypothesize that the number of micronodules might reflect the extent of lung involvement and those pulmonary function parameters might decrease. A previous study (14) showed that there were significant correlations between spirometric values and micronodules scores. The loss of pulmonary function in pulmonary sarcoidosis was found to be related to the amount of micronodules in HRCT (38). In our study, micronodule or macronodule scores did not correlate with pulmonary function indices. We speculate that these changes occurred only in a small area relative to the whole lung area; therefore, pulmonary function was not disturbed.
In this study, we found significant and negative correlations between consolidation, ground glass scores and PFT parameters. Airspace consolidation in sarcoidosis reflects the confluence of numerous micronodules that compress the surrounding alveoli or encroach on the alveolar spaces (6). The pathological significance of ground glass opacity can result from the confluence of multiple micronodular granulomas and/or fibrotic lesions (39). Ground glass and zones of consolidation are thought to be reversible processes; yet these changes may reveal fibrosis, which can lead to decreased gas diffusion and pulmonary volume (40). Although, some HRCT features might have the potential to discriminate between reversible disease (active inflammation) and irreversible disease (fibrosis), evaluating the reversibility of disease by HRCT appears to be possible only in retrospect with serial data (41). Zappala et al. (27) demonstrated that significant PFT trends correlate better with morphologic change as defined by serial HRCT scan than extent of disease on radiograph.
In general, our study suggests that newly diagnosed, advanced stage pulmonary sarcoidosis patients, confirmed via chest X-ray and/or lower PFT indices (e.g., <70% predicted), should be followed-up at shorter intervals.
The results of our study must be taken with caution, since it has several limitations. The first of all, these results may reflect the sarcoidosis manifestation of our patient population. One limitation of the design of our study relates to the relatively low number of patients who we investigated and the paucity of smokers. Moreover, there were no patients with stages 0 and IV sarcoidosis during the enrolment period and our interpretation of the data may be limited. Nevertheless, this was a prospective investigation and the study population was typical as in everyday practice. Another limitation inherent to this study is that we did not use any immunological or biochemical blood markers. However, to our knowledge, there are no good prognostic blood biomarkers for pulmonary sarcoidosis. The role of nuclear technique (radioisotope scintigraphy) in the follow-up of sarcoid patients is unknown.
Conclusions
In conclusion, this study supports the opinion that the staging of the pulmonary sarcoidosis with chest X-rays is still valuable from the prognostic point of view. Significant correlations between the radiologic stages of sarcoidosis and PFT parameters were found. Chest HRCT was significantly superior to chest X-ray in detecting mediastinal and pulmonary parenchymal changes important in the diagnostic stage of the examining patient. Tentatively, only consolidation and ground glass scores (neither of which are frequently found in sarcoidosis) hold prognostic value, since these were negatively correlated with PFT parameters. However, the role of HRCT needs to be better investigated evaluating serial examinations.
Acknowledgements
The authors gratefully acknowledge the language editing Audrūnas Gruslys.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: A signed informed consent form was obtained from each participant. The study was approved by the Vilnius Regional Biomedical Ethics Committee (No. 158200-12-559-160).
References
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:149-73.
- Greco FG, Spagnolo P, Muri M, et al. The value of chest radiograph and computed tomography in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2014;31:108-16.
- Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years' observation. Br Med J 1961;2:1165-72. [Crossref]
- Koyama T, Ueda H, Togashi K, et al. Radiologic manifestations of sarcoidosis in various organs. Radiographics 2004;24:87-104. [Crossref]
- Nunes H, Uzunhan Y, Gille T, et al. Imaging of sarcoidosis of the airways and lung parenchyma and correlation with lung function. Eur Respir J 2012;40:750-65. [Crossref]
- Criado E, Sánchez M, Ramírez J, et al. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics 2010;30:1567-86. [Crossref]
- Mostard RL, Verschakelen JA, van Kroonenburgh MJ, et al. Severity of pulmonary involvement and (18)F-FDG PET activity in sarcoidosis. Respir Med 2013;107:439-47. [Crossref]
- Rubini G, Cappabianca S, Altini C, et al. Current clinical use of 18FDG-PET/CT in patients with thoracic and systemic sarcoidosis. Radiol Med 2014;119:64-74. [Crossref]
- Vorselaars AD, Crommelin HA, Deneer VH, et al. Effectiveness of infliximab in refractory FDG PET-positive sarcoidosis. Eur Respir J 2015;46:175-85. [Crossref]
- Mohapatra PR, Vivek KU, Panigrahi MK, et al. Managing FDG PET-positive sarcoidosis: "a riddle wrapped in a mystery inside an enigma Eur Respir J 2016;47:346-7. [Crossref]
- Promteangtrong C, Salavati A, Cheng G, et al. The role of positron emission tomography-computed tomography/magnetic resonance imaging in the management of sarcoidosis patients. Hell J Nucl Med 2014;17:123-35.
- Grutters JC, Drent M, van den Bosch JM. Sarcoidosis. Eur Respir Mon 2009;46:126-54.
- F, Bouvry D, Freynet O, et al. Management of sarcoidosis in clinical practice. Eur Respir Rev 2016;25:141-50. [Crossref]
- Ors F, Gumus S, Aydogan M, et al. HRCT findings of pulmonary sarcoidosis; relation to pulmonary function tests. Multidiscip Respir Med 2013;8:8. [Crossref]
- Erdal BS, Crouser ED, Yildiz V, et al. Quantitative computerized two-point correlation analysis of lung CT scans correlates with pulmonary function in pulmonary sarcoidosis. Chest 2012;142:1589-97. [Crossref]
- Zappala CJ, Desai SR, Copley SJ, et al. Optimal scoring of serial change on chest radiography in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2011;28:130-8.
- Danila E, Jurgauskiene L, Malickaite R. BAL fluid cells and pulmonary function in different radiographic stages of newly diagnosed sarcoidosis. Adv Med Sci 2008;53:228-33. [Crossref]
- Danila E, Norkūniene J, Jurgauskiene L, et al. Diagnostic role of BAL fluid CD4/CD8 ratio in different radiographic and clinical forms of pulmonary sarcoidosis. Clin Respir J 2009;3:214-21. [Crossref]
- Naccache JM, Lavolé A, Nunes H, et al. High-resolution computed tomographic imaging of airways in sarcoidosis patients with airflow obstruction. J Comput Assist Tomogr 2008;32:905-12. [Crossref]
- Akira M, Kozuka T, Inoue Y, et al. Long-term follow-up CT scan evaluation in patients with pulmonary sarcoidosis. Chest 2005;127:185-91. [Crossref]
- Terasaki H, Fujimoto K, Müller NL, et al. Pulmonary sarcoidosis: comparison of findings of inspiratory and expiratory high-resolution CT and pulmonary function tests between smokers and nonsmokers. AJR Am J Roentgenol 2005;185:333-8. [Crossref]
- Drent M, De Vries J, Lenters M, et al. Sarcoidosis: assessment of disease severity using HRCT. Eur Radiol 2003;13:2462-71. [Crossref]
- Capelli A, Di Stefano A, Lusuardi M, et al. Increased macrophage inflammatory protein-1alpha and macrophage inflammatory protein-1beta levels in bronchoalveolar lavage fluid of patients affected by different stages of pulmonary sarcoidosis. Am J Respir Crit Care Med 2002;165:236-41. [Crossref]
- Abehsera M, Valeyre D, Grenier P, et al. Sarcoidosis with pulmonary fibrosis: CT patterns and correlation with pulmonary function. AJR Am J Roentgenol 2000;174:1751-7. [Crossref]
- Davies CW, Tasker AD, Padley SP, et al. Air trapping in sarcoidosis on computed tomography: correlation with lung function. Clin Radiol 2000;55:217-21. [Crossref]
- Remy-Jardin M, Giraud F, Remy J, et al. Pulmonary sarcoidosis: role of CT in the evaluation of disease activity and functional impairment and in prognosis assessment. Radiology 1994;191:675-80. [Crossref]
- Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest 2014;145:101-7. [Crossref]
- Gotway MB, Reddy GP, Webb WR, et al. High-resolution CT of the lung: patterns of disease and differential diagnoses. Radiol Clin North Am 2005;43:513-42. viii. [Crossref]
- Technical recommendations and guidelines for bronchoalveolar lavage (BAL). Report of the European Society of Pneumology Task Group. Eur Respir J 1989;2:561-85.
- Costabel U. CD4/CD8 ratios in bronchoalveolar lavage fluid: of value for diagnosing sarcoidosis? Eur Respir J 1997;10:2699-700. [Crossref]
- Lin YH, Haslam PL, Turner-Warwick M. Chronic pulmonary sarcoidosis: relationship between lung lavage cell counts, chest radiograph, and results of standard lung function tests. Thorax 1985;40:501-7. [Crossref]
- Drent M, Jacobs JA, de Vries J, et al. Does the cellular bronchoalveolar lavage fluid profile reflect the severity of sarcoidosis? Eur Respir J 1999;13:1338-44. [Crossref]
- Ziegenhagen MW, Rothe ME, Schlaak M, et al. Bronchoalveolar and serological parameters reflecting the severity of sarcoidosis. Eur Respir J 2003;21:407-13. [Crossref]
- Valeyre D, Soler P, Clerici C, et al. Smoking and pulmonary sarcoidosis: effect of cigarette smoking on prevalence, clinical manifestations, alveolitis, and evolution of the disease. Thorax 1988;43:516-24. [Crossref]
- Bergin CJ, Bell DY, Coblentz CL, et al. Sarcoidosis: correlation of pulmonary parenchymal pattern at CT with results of pulmonary function tests. Radiology 1989;171:619-24. [Crossref]
- Baydur A, Alsalek M, Louie SG, et al. Respiratory muscle strength, lung function, and dyspnea in patients with sarcoidosis. Chest 2001;120:102-8. [Crossref]
- Hamper UM, Fishman EK, Khouri NF, et al. Typical and atypical CT manifestations of pulmonary sarcoidosis. J Comput Assist Tomogr 1986;10:928-36. [Crossref]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68. [Crossref]
- Nishimura K, Itoh H, Kitaichi M, et al. Pulmonary sarcoidosis: correlation of CT and histopathologic findings. Radiology 1993;189:105-9. [Crossref]
- Murdoch J, Müller NL. Pulmonary sarcoidosis: changes on follow-up CT examination. AJR Am J Roentgenol 1992;159:473-7. [Crossref]
- Keijsers RG, van den Heuvel DA, Grutters JC. Imaging the inflammatory activity of sarcoidosis. Eur Respir J 2013;41:743-51. [Crossref]


