Current concepts in severe adult tracheobronchomalacia: evaluation and treatment
Expiratory central airway collapse (ECAC)
The tracheobronchial tree is a dynamic conduit that changes lumen size with exposure to varying pressures experienced during different phases of respiration. We will use the term ECAC to define the narrowing of the central airways during expiration and describe two different pathophysiologic entities, excessive dynamic airway collapse (EDAC) and tracheobronchomalacia (TBM). EDAC is a condition characterized by inward bulging of the atrophic muscular fibers in the posterior airway membrane during exhalation with narrowing of the cross sectional airway lumen, whereas TBM is characterized by weakness of the anterior tracheobronchial cartilage wall that may present with or without excessive dynamic invagination of the posterior membranous wall (1-3). Though airway malacia can present as an isolated segmental weakness of the trachea, or, less commonly of the bronchi alone, the focus of this review article is the severe, diffuse form of acquired TBM that affects the central airways more globally.
Severe TBM is an acquired condition, and progression to its diffuse form has been described by a study of bronchoscopic surveillance in patients with either isolated tracheomalacia or bronchomalacia in whom 67% and 100% of the patients, respectively, evolved to diffuse TBM during a mean follow-up interval of 5 years (4,5). This pathologic collapse of the posterior membrane produces dynamic outflow obstruction leading to symptoms such as dyspnea, orthopnea, intractable cough, and inability to clear secretions, predisposing the patient to recurrent infections (6,7). In addition, respiratory failure or failure of ventilator weaning may be the herald event that leads to a diagnosis of TBM (8,9).
TBM is an increasingly recognized abnormality of the central airways in patients with respiratory complaints, though its true incidence in the adult population as a whole remains to be elucidated. Estimates vary. For example, TBM has been identified in 1–4.5% of all patients undergoing bronchoscopy, but it has been reported to be present in more than 13% of patients who undergo evaluation for respiratory symptoms, and in as many as 23% of patients with a diagnosis of chronic bronchitis (6,10,11). One main reason for the wide range of estimates of the incidence of TBM in the general population is the ongoing debate over the definition of the extent of airway collapsibility and morphology required to meet the threshold for pathologic collapse. The currently accepted threshold for excessive airway collapse is a greater than 50% reduction in airway cross-sectional area with expiration (2,3). Under this definition, greater than 13% of patients with emphysema were found to have TBM on examination. When the threshold was raised to greater than 70% airway collapse, however, only 5% met the definition of TBM (12).
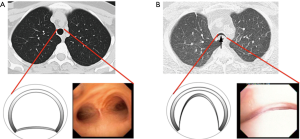
Recent work from our institution has demonstrated a wide range of expiratory tracheal collapsibility observed in healthy volunteers, with many asymptomatic individuals frequently exceeds the current diagnostic threshold for TBM (13,14). These observations indicate that there is physiologic range of dynamic airway collapse in the tracheobronchial tree and that the diagnosis of TBM should not be made solely by the identification of >50% expiratory collapse in the cross-sectional area of the airway lumen. In addition, it is important to emphasize that factor such age and gender should be considered when assessing forced expiratory airway collapse for suspected TBM (15). Consequently, when evaluating patients with suspected TBM are evaluated at the Complex Airway Disease Center of the Beth Israel Deaconess Medical Center (BIDMC) we restrict the categorization of severe TBM to patients with complete or near-complete collapse (>90%) of the central airways (Figure 1).
Etiology and classification of ECAC
The cause of diffuse TBM is often unknown, but it is frequently seen in patients with common respiratory conditions such as asthma, chronic bronchitis and emphysema. Most adults diagnosed with TBM have acquired forms of the disease, but there is a smaller subset of adult patients who have congenital tracheobronchomegaly (Mounier-Kuhn syndrome) which is not identified until adulthood (7). The etiology of acquired forms of TBM may be divided into inflammatory conditions, such as relapsing polychondritis (RP) and exposure to toxins (e.g., mustard gas) and compressive processes, including thyroid goiters, vascular abnormalities, and others (3,6,16). However, as previously mentioned, the etiology of TBM in most adult patients remains unknown.
The classification of ECAC has been the subject of multiple prior reviews (1-3,7) and is not the scope of this article. Several facts, however, are worth of mentioning. Malacic airways can be classified according to the morphology on inspiratory and expiratory images into three different subtypes: saber sheath-type TBM, circumferential-type TBM, and crescent-type TBM (17-19). Other authors have proposed subdividing airway collapse into two separate groups according to their mechanical etiology, distinguishing purely malacic airways from airways that only demonstrate membranous wall collapse as exhibiting either physiological collapse (dynamic airway collapse) or pathologic collapse (EDAC) (1,2). Although the physiology of airway mechanics is different for each entity, the clinical features of and therapeutic goals for TBM and excessive dynamic airway collapse are similar. Patients with severe excessive dynamic airway collapse refractory to medical management are candidates for surgical central airway stabilization to prevent excessive narrowing during exhalation.
We consider both airway cartilaginous wall and posterior membranous collapse as clinically significant forms of dynamic airway collapse. This is of particular relevance as the more pertinent consideration for clinical success of central airway stabilization seems to be less whether it is the cartilaginous or membranous wall pathology that potentiates collapse, but whether the presenting morphology is frown-shaped on expiration or lunate-shaped on inspiration, both of which are amenable to surgical stabilization.
Diagnostic interventions
The diagnostic evaluation of TBM can be challenging, as patients often have other concomitant chronic pulmonary conditions such as asthma or chronic obstructive pulmonary disease with overlapping symptoms. The mainstays of diagnosis are dynamic CT and dynamic bronchoscopy with forced expiratory maneuvers.
Dynamic flexible bronchoscopy (20) is currently the gold standard for diagnosing TBM since it permits real-time examination of the airways and accurately captures dynamic airway properties, with reproducible valid results in regard to information on morphology, degree, extent, and location of pathology (21). At BIDMC, patients undergo bronchoscopy under minimal sedation using intravenous midazolam and fentanyl to allow them to follow commands. Oral anesthesia is accomplished using 10–20 mL of 1% atomized lidocaine to suppress gag reflex. In addition, and 1% lidocaine in 2-mL aliquots are delivered through the bronchoscope during the procedure to irrigate the vocal cords, aryepiglottic folds, and entire tracheobronchial tree. The patients are placed in supine position. Typically, an Olympus BF P180 video bronchoscope (Olympus America, Melville, NY, USA) with a 4.9-mm outer diameter and 2.0-mm working channel is used to minimize any stenting effect. The bronchoscope is introduced into the proximal trachea at the level of the cricoid. At this point, patients are instructed to inhale deeply, hold it and then forcefully exhale (forced expiratory maneuver). Samples of imaging are obtained at six different locations in the tracheobronchial tree (proximal trachea, mid-trachea, distal trachea, right main stem bronchus, bronchus intermedius and left main stem bronchus) and evaluated for degree of luminal narrowing in the anteroposterior diameter (9,21).
In addition, dynamic flexible bronchoscopy facilitates the detection of coexisting pathology such as vocal cord abnormalities or bronchitis and permits biopsy of the mucosa as well as sputum sampling for histological and microbiologic analysis. Despite the inherent subjectivity of evaluating luminal collapse endoscopically, recently we have demonstrated a favorable inter- and intra-observer agreement in estimating degree of central airway collapse associated with TBM amongst pulmonologist from different institutions (20). However, expertise in this technique is not present nationwide.
Dynamic expiratory CT is a highly sensitive method for detecting airway malacia and has been shown to be concordant with dynamic flexible bronchoscopy (3,22); it is an effective, noninvasive test for diagnosing TBM. A prospective, multicenter trial comparing dynamic expiratory CT and dynamic bronchoscopy would be helpful to assess the reproducibility of our findings across multiple centers. Such a study will help to determine the prevalence of malacia among patients with chronic nonspecific respiratory symptoms and to assess the specificity of dynamic CT for diagnosing TBM. Both modalities are useful in describing the morphology, severity, and distribution of the disease. A caveat with either technique, however, is the potential variability in the effort of dynamic expiration, either because of inconsistent coaching of the forced expiratory maneuver or inconsistent compliance of patients with these instructions.
Pulmonary function testing is often unhelpful in screening patients during the initial diagnostic workup. A great proportion of patients may not achieve the predicted maximum forced expiratory flow while a substantial number of patients (21%) with moderate to severe TBM can demonstrate normal flow-volume loops (23). In our practice, the primary use of pulmonary function tests is to assess the degree of pulmonary comorbidity before intervention for TBM, and thus, the likelihood of an arduous or complicated recovery from tracheobronchoplasty.
Preoperative evaluation and stent trial
Once diagnosis of severe, diffuse TBM is established, and correlation of the deranged anatomy with significant symptoms and impaired quality of life is made, the patient is considered for treatment. Patients undergo physiologic assessment with pulmonary function testing and a 6-minute walk test. Standardized questionnaires are also administered to determine functional status (Karnofsky Performance Scale); symptomatology [modified Medical Research Council (mMRC) dyspnea scale]; and quality of life assessment [respiratory impacted quality of life (St. George Respiratory Questionnaire)] and Cough Specific Quality of Life Questionnaire (CQLQ) (9,16,21,24).
Our institutional protocol for preoperative evaluation of patients under consideration for tracheobronchoplasty is shown in Figure 2. Of note, it is important not only to confirm the extent and severity of TBM but also to maximize medical therapy for comorbidities such as obstructive airway disease. Routinely, patients undergo evaluation for vocal cord dysfunction and gastroesophageal reflux disease (GERD). If found, these diagnoses require adequate treatment prior to surgical evaluation. Previous observations from our group have revealed that GERD seems more prevalent in patients with TBM as compared with the general population and it may negatively impact the outcome of surgical tracheobronchoplasty (25,26). However, follow up studies are warranted to fully corroborate this correlation and thus, optimize the evaluation approach for patients with diffuse TBM.
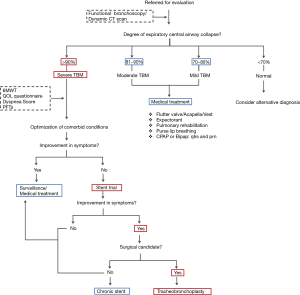
At BIDMC, a Y-shaped silicone tracheobronchial stent is placed in advance of a patient’s assessment for candidacy for surgical intervention (9). The stent is deployed within the airway portions that would be stabilized by a surgical tracheobronchoplasty—namely the thoracic trachea and left and right main stem bronchi, but not the cervical trachea, distal lobar or segmental bronchi, or smaller airways. After a two week trial, patients are evaluated for the level of symptomatology improvement attributable from stabilization of the central airways. Improvement of dyspnea is the most consistent metric of treatment effect, likely because dyspnea is the most common presenting complaint in patients with diffuse TBM and because of the immediate effect of the stent on expiratory airflow.
Notably, iatrogenic symptoms related to the stent may develop and detract from the perceived subjective sense of benefit that the patient might have with stent stabilization of the airway. Mucus plugging may occur in up to 36% of patients, and this might obscure the patient’s subjective improvement in dyspnea (9,16). The stent may ease the barking cough associated with TBM, but this may be substituted by a cough secondary to the irritating effect of a foreign body in the airway. In similar equivocal fashion, secretions may be expectorated more easily with the stent in place, but the volume of sputum production might be increased by the presence of the stent within the airway lumen. In some instances, the stent trial may prove inadequate or impossible to complete in patients with unusually large or small airways if correct stent sizes is not available, or in patients in whom their anatomy impedes rigid bronchoscopy for Y-stent placement (e.g., extreme cervical kyphosis). To lessen these potential confounding outcomes, our group recently has piloted the use of uncovered self-expanding metallic stents for short-term trials (24,27). Despite these limitations, a planned short-term (<2 weeks) stent trial still may yield useful information about those patients with diagnosed TBM who may respond best to surgery. In our experience, between 60% and 75% of patients with TBM will respond to the stent trial in positive fashion, and improvement in symptoms, quality of life and exercise capacity following definitive surgical airway stabilization with tracheobronchoplasty is seen in 80% of these selected patients (21,25).
Tracheobronchoplasty: technical aspects and surgical results
The objective of surgical management is to stabilize the membranous wall of the intrathoracic trachea, both mainstem bronchi and bronchus intermedius. The redundant posterior membranous wall is plicated and fixed to the posterior splint by suturing a knitted polypropylene mesh to reconstitute the airway morphology and prevent intrusion of the membranous wall into the lumen of the airway (Figure 3). With time, the mesh is incorporated by fibrosis, with subsequent stiffening of the posterior membranous wall. The suture placement is also carefully selected to achieve tension across the membranous wall. The operation has historical roots going back over 50 years (16,29) and the BIDMC technique has been described in detail elsewhere (25). It is worth noting that concentric forms of ECAC do not respond to this posterior stabilization.
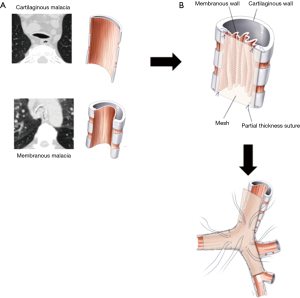
In brief, left lung ventilation is established using a modified double-lumen endobronchial tube created by shaving off the tracheal lumen before placement in order to decrease airway distortion caused by the endotracheal tube. The patient is placed in left lateral decubitus position, and a right poster lateral thoracotomy is performed. The azygos vein is ligated and divided and the posterior membranous walls of the thoracic trachea and mainstem bronchi are exposed. The right vagus nerve is dissected free and preserved. Care is taken not to entrap the nerve with the mesh or sutures once the posterior plication is begun. The posterior membrane of the trachea is dissected free of any adventitial attachments all the way to the edge of the cartilaginous-membranous junctions bilaterally. To prevent ischemia, aggressive dissection past these junctions anteriorly onto the lateral walls of the trachea is avoided. The transverse airway diameter is measured posteriorly at the proximal and distal trachea, right main stem bronchus, bronchus intermedius, and left main stem bronchus. A 2×12-inch knitted polypropylene mesh (Ref Number 0112670; C. R. Bard, Inc., Murray Hill, NJ, USA) is fashioned into a Y-shaped posterior splint based on these measurements. A 0.5-cm margin is left on the edge of the Y-shaped mesh to allow a secure, unfrayed material through which to suture. The mesh is then secured using rows of four partial thickness interrupted sutures 4-0 Prolene (Ethicon, Johnson & Johnson, Gateway; Cincinnati, OH, USA) across the width of the trachea from the mesh strip to the tracheal wall. Typically, the entire thoracic trachea is splinted first, followed by the right mainstem and bronchus intermedius, and finally the left mainstem bronchus. In our experience, it is not sufficient simply to use the sutures to affix the mesh to the posterior membrane. The mesh is inherently ‘floppy’, so adding it as a simple reinforcement layer to the malacic airway does not yield the optimal amount of stabilization. Rather, careful selection of the configuration of the individual sutures helps create tension across the airway by keeping the mesh very taut. This process requires row to row adjustments of the sutures, keeping a balance between creating tension and excessively narrowing the airway. Once the posterior splinting is completed, the pleural space is irrigated, and a chest drain is placed. After placement, the endobronchial tube is pulled back into the trachea and flexible bronchoscopy through the tube is performed to assure that an acceptable anatomy has resulted from the tracheobronchoplasty. Occasionally, bronchoscopy can be utilized during suture placement to optimize tension and surveil for full-thickness sutures. After closure of the thoracotomy, the patient is reintubated with a larger single-lumen endotracheal tube to allow aspiration bronchoscopy before extubation.
We have previously reported our experience with a cohort of 218 patients referred for evaluation at BIDMC from 2002 to 2009 (21). Of these, 161 patients were diagnosed by dynamic airway CT and bronchoscopy with severe, diffuse TBM and underwent stenting trial. On follow-up, 99 of these stented patients had amelioration of their symptoms, and 63 underwent tracheobronchoplasty. There were 28 women (44%) in the cohort, and the mean age was 59 years (SD±12.5; range, 35–82 years). Preoperative comorbidities included respiratory conditions, such as chronic obstructive pulmonary disease (37%), asthma (23%), and Mounier-Kuhn syndrome (8%). Notably, GERD was comorbidity present in 48% of patients. The operation time averaged 373 minutes (SD±93; range, 180–635 minutes). The median hospital length of stay was 8 days (range, 4–92 days), and the median length of intensive care unit stay was 3 days (range, 0–91 days). Complications were seen in 38% of patients, including two deaths (3.2%), both during our original 2002-2005 cohort experience. One patient succumbed to acute worsening usual interstitial pneumonia and the other to a massive pulmonary embolism. Postoperative morbidity included new postoperative respiratory infection in 14 patients (22%), atrial arrhythmia in 6 (10%), acute renal failure (creatinine >2) in 4 (6%), unplanned return to the intensive care unit in 3 (5%), urinary tract infection in 2 (3%), pulmonary embolism in 2 (3%), myocardial infarction and cardiomyopathy in 1 patient (2%), and wound infection in 1 (2%). Reintubation was necessary in 6 patients (10%). A tracheotomy was placed in 9 patients (14%), including four tracheotomies performed intraoperatively immediately after the tracheobronchoplasty was completed in anticipation of the need for frequent aspiration bronchoscopy and tracheal suctioning. Of these patients, five were decannulated successfully. One patient still had his tracheotomy at the 3-month postoperative visit and was subsequently lost to follow up; one patient was lost to follow-up immediately after his TBP. The two patients who died postoperatively both died with tracheotomies in place. Neither reintubation nor tracheotomy was required in the latter half of the series. There were no reoperations for bleeding. Aspiration bronchoscopy was used frequently in the postoperative period (16,25,30).
Surgical stabilization of the malacic tracheal and bronchial airway has yielded significant improvements in respiratory-related quality of life (St George Respiratory questionnaire), dyspnea indices [Baseline/transition dyspnea index, American Thoracic Society (ATS) Dyspnea Score], performance status (Karnofsky score), and exercise tolerance (6-minute walk test) Table 1. Furthermore, central airway stabilization has shown a significant reduction in the mean percentage of expiratory tracheal collapse in malacic airways following tracheobronchoplasty (3,22). Although it is possible to show anatomic improvement central airway collapsibility on dynamic bronchoscopy and CT scan, enhancement of “end organ” effects, such as decreased air trapping have not been demonstrated (3,21). It is uncertain whether the lack of change in air trapping in our entire cohort is due to chronic, irreversible small airways disease from recurrent infections or comorbid conditions (e.g., COPD, asthma), or if a longer time period of follow-up is necessary to document changes.

Full table
Surveillance and long-term outcomes
Two distinct considerations should be taken into account when planning patient care following tracheobronchoplasty. First, adequate surveillance should be ensured to manage the long-term complications related to TBP itself. This should include a multidisciplinary collaborative effort including experienced thoracic surgeons, interventional pulmonologists, otorhinolaryngologists and gastroenterologists. Second, a surveillance program should be considered to detect recurrences of symptoms and/progression of disease to cervical trachea or lobar bronchi early enough to allow potentially curative retreatment. At BIDMC, we follow patients post operatively with routine dynamic bronchoscopy and CT surveillance starting at 3 months post operatively to establish a new baseline and then yearly thereafter. In addition respiratory-related quality of life, performance status, and exercise tolerance are measured at these intervals.
Although the long-term morbidity and durability of TBP needs further study, our group has made several recent observations on long-term outcomes. Improvement of symptoms was reported in 77.8% (n=90) at 3 months, 75% (n=56) at 1 year, 67.6% (n=37) at 2 years and 65% (n=20) at 5 years. Airway patency was documented at follow-up dynamic bronchoscopy in 94.6% (n=90) at 3 months, 85.7% (n=56) at 1 year, 91.4% (n=35) at 2 years and 100% (n=19) at 5 years. Long-term complications have included chronic pain (17%), recurrence of disease requiring redo TBP (8%), dysphagia (9%), and mesh erosion (3%) (31).
Additional treatment and novel therapies
Airway oscillatory devices (flutter valve) or external percussion vests used twice or three times per day can serve as an adjunct for airway clearing therapy. Pneumatic splinting using nasal continuous positive airway pressure (CPAP) has been described as an alternative therapy with variable success; yet, it requires an external device difficult to use with daily activities (32). Robotically assisted bilateral bronchoplasty, bioresorbable three-dimensional airway stents, endobronchial laser therapy, and tracheal cartilage regeneration techniques are under investigation and further studies are warranted to determine their safety and efficacy (33-35).
Conclusions
Severe, diffuse TBM and EDAC in the adult population are conditions that affect the central airways and often result in dyspnea, intractable cough, inability to clear secretions, and recurrent infections. Initial evaluation is done with dynamic bronchoscopy and CT scanning. Pulmonary function tests are not diagnostic but help to stratify patients during the initial evaluation. Once severe (>90%) collapse of the airways is observed, further investigations are required to determine if airway stabilization will improve symptoms. Short-term airway stabilization with tracheobronchial stenting in patients with severe symptomatic TBM serves as a surrogate for the effects of surgery, though frequent side effects and complications unique to the stent trial may be seen. Surgical stabilization of the central malacic airways by posterior splinting with a prosthetic mesh (tracheobronchoplasty) results in significant symptomatology improvement, health-related quality of life, as well as functional and exercise capacity in carefully selected patients. Long-term anatomical improvement may be achieved, but close surveillance is necessary to detect recurrence or progression of malacia. As our experience with the treatment of TBM increases, we hope to refine the assessment tools that will help us understand which patients truly benefit from this highly technical operation. Close coordination of a multidisciplinary team of dedicated radiologists, interventional pulmonologists, and thoracic surgeons helps ensure optimal selection and treatment of these challenging patients.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflict of interest to declare.
References
- Murgu S, Colt H. Tracheobronchomalacia and excessive dynamic airway collapse. Clin Chest Med 2013;34:527-55. [Crossref]
- Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology 2006;11:388-406. [Crossref]
- Ridge CA, O'donnell CR, Lee EY, et al. Tracheobronchomalacia: current concepts and controversies. J Thorac Imaging 2011;26:278-89. [Crossref]
- Nuutinen J. Acquired tracheobronchomalacia. A bronchological follow-up study. Ann Clin Res 1977;9:359-64.
- Nuutinen J. Acquired tracheobronchomalacia. A clinical study with bronchological correlations. Ann Clin Res 1977;9:350-5.
- Jokinen K, Palva T, Sutinen S, et al. Acquired tracheobronchomalacia. Ann Clin Res 1977;9:52-7.
- Carden KA, Boiselle PM, Waltz DA, et al. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest 2005;127:984-1005. [Crossref]
- Collard P, Freitag L, Reynaert MS, et al. Respiratory failure due to tracheobronchomalacia. Thorax 1996;51:224-6. [Crossref]
- Ernst A, Majid A, Feller-Kopman D, et al. Airway stabilization with silicone stents for treating adult tracheobronchomalacia: a prospective observational study. Chest 2007;132:609-16. [Crossref]
- Jokinen K, Palva T, Nuutinen J. Chronic bronchitis. A bronchologic evaluation. ORL J Otorhinolaryngol Relat Spec 1976;38:178-86. [Crossref]
- Ikeda S, Hanawa T, Konishi T, et al. Diagnosis, incidence, clinicopathology and surgical treatment of acquired tracheobronchomalacia. Nihon Kyobu Shikkan Gakkai Zasshi 1992;30:1028-35.
- Ochs RA, Petkovska I, Kim HJ, et al. Prevalence of tracheal collapse in an emphysema cohort as measured with end-expiration CT. Acad Radiol 2009;16:46-53. [Crossref]
- Boiselle PM, O'Donnell CR, Bankier AA, et al. Tracheal collapsibility in healthy volunteers during forced expiration: assessment with multidetector CT. Radiology 2009;252:255-62. [Crossref]
- Litmanovich D, O'Donnell CR, Bankier AA, et al. Bronchial collapsibility at forced expiration in healthy volunteers: assessment with multidetector CT. Radiology 2010;257:560-7. [Crossref]
- O'Donnell CR, Litmanovich D, Loring SH, et al. Age and sex dependence of forced expiratory central airway collapse in healthy volunteers. Chest 2012;142:168-74. [Crossref]
- Gangadharan SP. Tracheobronchomalacia in adults. Semin Thorac Cardiovasc Surg 2010;22:165-73. [Crossref]
- Lomasney L, Bergin CJ, Lomasney J, et al. CT appearance of lunate trachea. J Comput Assist Tomogr 1989;13:520-2. [Crossref]
- Boiselle PM, Ernst A. Tracheal morphology in patients with tracheomalacia: prevalence of inspiratory lunate and expiratory "frown" shapes. J Thorac Imaging 2006;21:190-6. [Crossref]
- Boiselle PM, Feller-Kopman D, Ashiku S, et al. Tracheobronchomalacia: evolving role of dynamic multislice helical CT. Radiol Clin North Am 2003;41:627-36. [Crossref]
- Majid A, Gaurav K, Sanchez JM, et al. Evaluation of tracheobronchomalacia by dynamic flexible bronchoscopy. A pilot study. Ann Am Thorac Soc 2014;11:951-5. [Crossref]
- Ernst A, Odell DD, Michaud G, et al. Central airway stabilization for tracheobronchomalacia improves quality of life in patients with COPD. Chest 2011;140:1162-8. [Crossref]
- Lee KS, Ashiku SK, Ernst A, et al. Comparison of expiratory CT airway abnormalities before and after tracheoplasty surgery for tracheobronchomalacia. J Thorac Imaging 2008;23:121-6. [Crossref]
- Majid A, Sosa AF, Ernst A, et al. Pulmonary function and flow-volume loop patterns in patients with tracheobronchomalacia. Respir Care 2013;58:1521-6. [Crossref]
- Majid A, Alape D, Kheir F, et al. Short-Term Use of Uncovered Self-Expanding Metallic Airway Stents for Severe Expiratory Central Airway Collapse. Respiration 2016;92:389-96. [Crossref]
- Gangadharan SP, Bakhos CT, Majid A, et al. Technical aspects and outcomes of tracheobronchoplasty for severe tracheobronchomalacia. Ann Thorac Surg 2011;91:1574-80; discussion 1580-1. [Crossref]
- Nataraj D, Majid A, Chuttani R. Prevalence of Gastroesophageal Reflux in Patients with Tracheobronchomalacia. Chest 2009;136:80s. [Crossref]
- Ochoa S, Cheng GZ, Folch E, et al. Use of Self-expanding Metallic Airway Stents in Tracheobronchomalacia. J Bronchology Interv Pulmonol 2015;22:e9-e11. [Crossref]
- Dienemann HC, Hoffmann H, Detterbeck FC. Chest Surgery, Springer Surgery Atlas Series. Springer-Verlag Berlin Heidelberg 2015.
- Herzog H. Stabilization of the trachea and the main bronchi on prolapse of the pars membranacea. Scand J Respir Dis Suppl 1972;80:81-99.
- Majid A, Guerrero J, Gangadharan S, et al. Tracheobronchoplasty for severe tracheobronchomalacia: a prospective outcome analysis. Chest 2008;134:801-7. [Crossref]
- Alape DE, Gangadharan S, Folch E, et al. Tracheobronchoplasty for Severe Tracheobronchomalacia: Short and Long-Term Outcomes. Am J Respr Crit Care Med 2016;193:A3386.
- Ferguson GT, Benoist J. Nasal continuous positive airway pressure in the treatment of tracheobronchomalacia. Am Rev Respir Dis 1993;147:457-61. [Crossref]
- Morrison RJ, Hollister SJ, Niedner MF, et al. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci Transl Med 2015;7:285ra64. [Crossref]
- Lazar JF, Posner DH, Palka W, et al. Robotically Assisted Bilateral Bronchoplasty for Tracheobronchomalacia. Innovations (Phila) 2015;10:428-30. [Crossref]
- Dutau H, Maldonado F, Breen DP, et al. Endoscopic successful management of tracheobronchomalacia with laser: apropos of a Mounier-Kuhn syndrome. Eur J Cardiothorac Surg 2011;39:e186-8. [Crossref]


