Changes in the action potential and transient outward potassium current in cardiomyocytes during acute cardiac rejection in rats
Introduction
Cardiac transplantation has become an established therapeutic option for eligible patients with end-stage cardiac diseases. However, acute cardiac rejection remains a frequent complication that can severely threaten the patient’s survival in the early days after cardiac transplantation (1,2). Earlier research has revealed that the ventricular voltage is decreased (3,4), the intramyocardial impedance is increased (5), the resting potential (RP) and transmembrane action potential (AP) amplitude of the ventricular muscle is decreased (6), and the QRS amplitude of the autonomous intramyocardial electrograms (IMEG) and the maximum slope of the descending T wave of the ventricular evoked response is decreased during acute cardiac rejection in experimental models (7). All of these suggest that the electrophysiological characteristics of cardiomyocytes could be changed during acute cardiac rejection. However, the mechanism of electrophysiologic changes in the cardiomyocytes is still unclear. The AP is a sign of cells in an exited state, and the modification of the shape and duration of the AP could lead to changes in the electrophysiological properties of cardiomyocytes. The transient outward potassium current (Ito) is the main component of the AP repolarization phase 1 in myocardial cells. The modification of the current amplitude and kinetic characteristics can significantly change the AP and other ionic conductances (8,9). The characteristics of the AP and Ito in cardiomyocytes during acute cardiac rejection have not yet been established. To gain more insight into the electrophysiological changes and their possible ionic mechanism, our study was designed to investigate the changes in the AP and Ito in cardiomyocytes in an abdominal heart transplantation model in rats during acute cardiac rejection.
Methods
Animals
Eight-week-old male Brown Norway (BN) rats (n=60) and Lewis rats (n=20) with an average body weight of 230 g (200–250 g) were obtained from the experimental animal center of the Beijing Anzhen Hospital, Capital Medical University. Forty BN rats were used as donors, and 20 BN rats and 20 Lewis rats were used as recipients. Food and water were provided by placing food pellets and water bottles on a grid located on top of the tank. The water in the tank was changed daily until the experimental endpoints.
All surgical interventions and postoperative animal care were performed in accordance with the National Institutes of Health Guide-lines for the Care and Use of Laboratory Animals (National Research Council, 1996, USA) and were approved by the Chinese National Committee on the Use of Experimental Animals for Medical Purposes, Beijing Branch. All procedures were performed on animals in an unconscious state. All efforts were made to minimize the number of animals used and their suffering.
Heterotopic heart transplantation
Heterotopic cardiac transplantation was performed as described by Ono and Lindsey (10), with some modifications as described elsewhere (11). The rats were anesthetized using 2% phenobarbital sodium (50 mg/Kg). The average cold ischemic time was less than 45 min. The graft function was assessed daily via abdominal palpation by a single investigator. The cessation of a palpable cardiac contraction was defined as graft loss, which was confirmed after laparotomy. When graft loss occurred before their experimental endpoints for various reasons, new matched rats were introduced to maintain a constant number of recipient animals within each group.
Experimental design
The 20 allogeneic recipient rats (BN-to-Lewis) and 20 isogeneic recipient rats (BN-to-BN) were randomly separated into 4 groups respectively (n=5). Five grafted hearts were randomly harvested for pathological examination, and another five grafted hearts were harvested for electrophysiological examination at the 2nd day and the 4th day after the operation.
Pathological examination
At the 2nd day and the 4th day after the operation, a laparotomy was performed under intraperitoneal anesthesia by 2% pentobarbital sodium and the grafted heart was harvested while still beating, fixed with 10% phosphate buffered formalin, and embedded in paraffin. The specimen was sliced into samples 3 or 4 µm in thickness and then stained with hematoxylin and eosin (HE). One investigator, in a blind fashion, routinely evaluated the stained sections (HE) of grafted hearts with a semiquantitative scale for myocyte loss and the degree of inflammation [compatible with the ISHLT rejection grades of human allografts (12)], in which 0 indicates no inflammation and myocyte loss (ISHLT grade 0); 1 indicates perivascular inflammation (ISHLT grade 1A); 2 indicates interstitial inflammation (ISHLT grade 1B); 3 indicates inflammation with focal myocyte loss (ISHLT grade 2); 4 indicates inflammation with multifocal myocyte loss (ISHLT grade 3A); 5 indicates inflammation with confluent foci of myocyte loss (ISHLT grade 3B); and 6 indicates inflammation with large areas of necrosis (>25% myocyte loss) and/or necrotizing vasculitis (ISHLT grade 4) (13,14).
Electrophysiological measurements
Solution
A Tyrode’s solution was prepared containing (in mM/L): NaCl 126, KCl 5.4, MgCl2 1, CaCl2 1.8, NaH2PO4 0.33, glucose 10 and 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 10, and the pH was adjusted to 7.4 with NaOH. A Ca2+-free Tyrode’s solution was prepared by removing CaCl2 from the aforementioned Tyrode’s solution.
The enzyme solution used for the rat cardiomyocyte isolation contained 0.1 g/L collagenase (type II) and 0.5 g/L BSA in Tyrode’s solution.
For recording the Ito current, the pipettes were filled with (in mM/L): K-aspartame acid 140, MgATP 4, MgCl2 1, ethylene glycol tetraacetic acid (EGTA) 10, guanosine triphosphate (GTP) 0.1, HEPES 10, and the pH was adjusted to 7.3 with KOH.
For recording Ito currents, the myocytes were superfused with a solution containing (in mM/L): NaCl 140, KCl 4, MgCl2 1, CaCl2 1, HEPES 10, glucose 5, with the pH adjusted to 7.4 with NaOH. Tetrodotoxin inhibits the Na+ current; CdCl2 inhibits the Ca2+ current.
Cell preparations
A single cardiomyocyte was dissociated by an enzymatic dissociation as described previously. Grafted hearts were excised rapidly after the recipient rats were given an intraperitoneal injection of 2% pentobarbital sodium (50 mg/Kg). The hearts were perfused retrogradely on a Langendorff apparatus with a Ca2+-free Tyrode’s solution after an incision was made in the right atrium. Five minutes later, the perfusate was switched to an enzymatic solution for fifteen minutes. The perfusates were bubbled with 95% O2 + 5% CO2 and maintained at 37 °C. The ventricles were cut into small chunks and gently agitated in Ca2+-free Tyrode’s solution. The myocytes were filtered through a nylon mesh (pore size 200 µm) and stored in Ca2+-free Tyrode’s solution at 4 °C.
Patch clamp experiments
Quiescent, calcium-tolerant, rod-shaped cells with a clear cross-striation were used for the AP recordings at 35 °C. The transmembrane potentials and currents were recorded using the whole-cell patch-clamp technique with an EPC10 amplifier (HEKA Instruments, Germany). All signals were acquired at 5 kHz and analyzed by Patchmaster version 2.72 software (HEKA Instruments, Germany). The whole-cell currents and APs, obtained under voltage clamp, were filtered at 1–5 kHz and sampled at 5–50 kHz, and the series resistance was typically <5 mega ohms after approximately 70% compensation.
The APs were elicited using the current-clamp mode at a rate of 5.0 Hz of 30-train suprathreshold current pulses. The cardiomyocytes were electrically stimulated by intracellular current injection through patch electrodes using depolarizing pulses with duration of 5 ms and an amplitude of 1,500 pA. The action potential duration (APD) was measured at 50% and 90% of repolarization (APD50 and APD90). Ito was recorded using the voltage-clamp mode. We used a pre-pulse to –40 mV for 50 ms to inactivate INa. Ito was recorded in voltage-clamp mode with 300-ms pulses from a holding potential of –90 mV, with different test potentials increasing from –40 mV to +60 mV with 10-mV steps.
Statistical analysis
All data are presented as the mean ± SD. The curves were fitted with Origin 6.0. The statistical significance was determined using a t-test to compare multiple groups. Statistical significance was defined by a P value <0.05.
Results
Heterotopic heart transplantation
All recipients survived excepted one recipient in allogeneic group died of anastomotic bleeding 6 hours after the operation. New matched rats (BN-to-Lewis) were introduced in the allogeneic group. And the grafted hearts were all beating before they were heavested.
Histology and rejection score of grafted heart
The histopathological findings showed an outer-to-inner lymphoplasmocytic infiltration; there was perivascular and interstitial inflammation at the 2nd day after the operation. Inflammation with multifocal myocyte loss and confluent foci of myocyte loss could be found across the ventricular wall at the 4th day (Figure 1, Table 1).
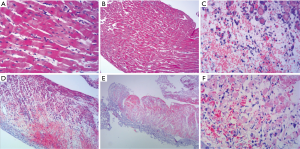
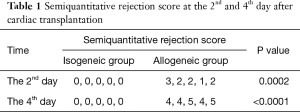
Full table
AP changes during acute cardiac rejection
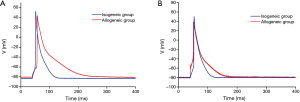
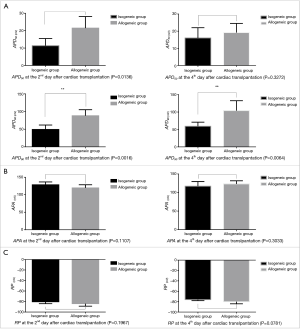
The APD90 of cardiomyocytes was significantly prolonged in the allogeneic group at the 2nd day (P<0.0016) and 4th day (P<0.0064) after cardiac transplantation. There was no significant difference in action potential amplitude (APA) or RP (Tables 2,3 and Figures 2,3).
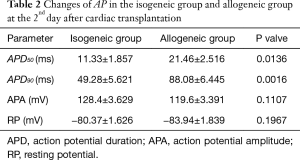
Full table
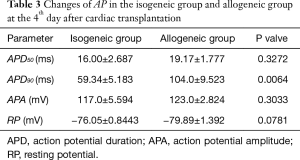
Full table
Ito current changes during acute cardiac rejection
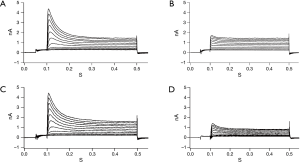
There was no significant difference in the current density of Ito at the 2nd day after cardiac transplantation between the isogeneic group and allogeneic group. At the 4th day, the Ito density of the allogeneic group was significantly decreased compared to the isogeneic group (P<0.01) (Figures 4,5).

Discussion
So far, the changes of the cardiomyocyte AP and the possible ionic mechanism during acute cardiac rejection in rats are not yet clear. In the present study, we first established a stable cardiac rejection model in allogeneic rats because the results obtained from cardiac transplantation studies in animal models must depend upon the model species involved, and particularly upon the stage of the rejection process studied (6). The isogeneic model was also developed to eliminate the influence of the operation, anesthesia, cold ischemic preservation and withdrawal of autonomic nervous system control. On the 2nd day and the 4th day after the operation, the isogeneic group showed no evidence of rejection, while the allogeneic group showed mild to moderate cardiac rejection without cardiomyocyte loss or necrosis. So the model of BN-to-Lewis heterotopic heart transplantation was suitable for electrophysiological studies in the early stage of acute cardiac rejection.
The cardiomyocyte AP is the most fundamental component of cardiac electrophysciology. The AP shape and APD are the most important features in AP. The present study showed that the APD90 of cardiomyocytes was significantly prolonged during mild to moderate cardiac rejection, which suggested that the increased APD of cardiomyocytes may be a cellular mechanism underlying the electrophysiological changes. Binah et al. (15) found the APA, RP, and maximum rate of depolarization were clearly decreased in ventricular myocytes in the heterotopically transplanted rat heart using the standard microelectrode recording technique. However, Babuty et al. (4) observed that the APD and APA recorded from the right and left papillary muscles of the allogeneic heart significantly increased during early rejection in rats. Maybe the stage of the rejection process and the species involved in the rejection model led to the difference. However, there are no more detailed studies that could precisely characterize the electrophysiological changes in cellular preparations. In the present study, the grafted hearts in the allogeneic group and isogeneic group all underwent the changes that take place in the abdominal cavity, such as fibrin layer formation, ascites, intestinal adhesion, and myocardial edema (16). We found that there was no significant difference in the APA and RP between the allogeneic group and isogeneic group. So the prolongation of the APD may be the basic electrophysiological change in cardiomyocytes during the early stage of acute cardiac rejection. The changes would be expected to result in depressed conduction of the cardiac impulse through the ventricles, which would make contractile function less synchronous (17). Many studies have demonstrated that electrophysiological abnormalities are closely connected with acute cardiac rejection, including rejection-related arrhythmias in the heart transplant patient (18), decreased ventricular voltage (3,4), increased intramyocardial impedance (5) and the characteristic changes in the IMEG (7,19).
The AP of a cardiomyocyte is generated by highly coordinated changes in different ionic conductances. Ito is a transient outward potassium current, which is rapidly activated and inactivated in response to the initial phase of the cardiomyocyte AP, resulting in repolarization. Ito is the key outward potassium current in the early stage of the AP, and the alterations of Ito would be expected to alter the morphology and duration of the AP (20). It has been confirmed that Ito is important in physiological and pathophysiological processes. In the present study, the current density of Ito was significantly decreased at the 4th day in the allogeneic group compared with the isogeneic group after cardiac transplantation, which may explain the prolongation of the APD in cardiomyocytes during acute cardiac rejection. Additionally, more evidence has shown that Ito has kinetic properties that make it especially effective in modulating the cardiac exitation-contraction coupling through its influence on ICa,L, and possibly ICa,NCX (8,9,17,20). As a result, modification of Ito eventually leads to the impairment of cardiac systolic and diastolic function during acute cardiac rejection. The present study also observed that the cardiomyocyte APD in the allogeneic group was significantly prolonged at the 2nd day, while there was no significant difference in the current density of Ito between the two groups. The results indicate that some other factors may act on the Ito. Further studies are necessary to identify the mediators of the Ito in cardiomyocytes during acute cardiac rejection. An understanding of the electrophysiological mechanisms of cardiomyocytes could improve the diagnosis and treatment of acute cardiac rejection.
In conclusion, we suggest that the prolongation of the APD and the decrease of the Ito current density in cardiomyocytes might be basic electrophysiological mechanisms in the early stage of acute cardiac rejection in rats. The prolongation of the APD might be attributed to the decreased current densities of Ito.
Limitations
We only focused on the changes of current density of Ito and did not investigate other ion channels such as those that give rise to Ca2+ currents, which are also involved in the duration of AP. Whether Ca2+ currents or other currents exert the same effect on APD during acute cardiac transplantation in rats requires further investigation.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China (No. 81270215).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Chinese National Committee on the Use of Experimental Animals for Medical Purposes, Beijing Branch (No. 29971) and written informed consent was obtained from all patients.
References
- Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Heart Transplantation Report--2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant 2015;34:1244-54. [Crossref]
- Dipchand AI, Rossano JW, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Eighteenth Official Pediatric Heart Transplantation Report--2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant 2015;34:1233-43. [Crossref]
- Avitall B, Payne DD, Connolly RJ, et al. Heterotopic heart transplantation: electrophysiologic changes during acute rejection. J Heart Transplant 1988;7:176-82.
- Babuty D, Aupart M, Cosnay P, et al. Electrocardiographic and electrophysiologic properties of cardiac allografts. J Cardiovasc Electrophysiol 1994;5:1053-63. [Crossref]
- Pfitzmann R, Müller J, Grauhan O, et al. Intramyocardial impedance measurements for diagnosis of acute cardiac allograft rejection. Ann Thorac Surg 2000;70:527-32. [Crossref]
- Babuty D, Ojeda C, Machet MC, et al. Severe and early alteration of action potential during acute cardiac rejection in rats. J Cardiovasc Electrophysiol 1998;9:1085-93. [Crossref]
- Shi J, Qian S, Meng X, et al. Reliability of intramyocardial electrogram for the noninvasive diagnosis of acute allograft rejection after heart transplantation in rats. J Thorac Dis 2014;6:126-33.
- Sah R, Ramirez RJ, Oudit GY, et al. Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (I(to)). J Physiol 2003;546:5-18. [Crossref]
- Cordeiro JM, Calloe K, Aschar-Sobbi R, et al. Physiological roles of the transient outward current Ito in normal and diseased hearts. Front Biosci (Schol Ed) 2016;8:143-59. [Crossref]
- Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg 1969;57:225-9.
- Ruzza A, Vespignani R, Czer LS, et al. Heterotopic heart transplantation in rats: improved anesthetic and surgical technique. Transplant Proc 2010;42:3828-32. [Crossref]
- Billingham ME, Cary NR, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant 1990;9:587-93.
- Farivar AS, McCourtie AS, MacKinnon-Patterson BC, et al. Poly (ADP) ribose polymerase inhibition improves rat cardiac allograft survival. Ann Thorac Surg 2005;80:950-6. [Crossref]
- Szabolcs MJ, Sun J, Ma N, et al. Effects of selective inhibitors of nitric oxide synthase-2 dimerization on acute cardiac allograft rejection. Circulation 2002;106:2392-6. [Crossref]
- Binah O, Zhang HL, Oluwole SF, et al. Mechanical and electrophysiologic changes in rat cardiac allografts during immunologic rejection. Transplantation 1991;52:508-12. [Crossref]
- Castejon R, Cabo J, Gamallo C, et al. Electrophysiological and anatomical findings in heart transplantation: experimental study. Pacing Clin Electrophysiol 1990;13:845-51. [Crossref]
- Bassani RA. Transient outward potassium current and Ca2+ homeostasis in the heart: beyond the action potential. Braz J Med Biol Res 2006;39:393-403. [Crossref]
- Hamon D, Taleski J, Vaseghi M, et al. Arrhythmias in the Heart Transplant Patient. Arrhythm Electrophysiol Rev 2014;3:149-55. [Crossref]
- Knosalla C, Grauhan O, Muller J, et al. Intramyocardial electrogram recordings (IMEG) for diagnosis of cellular and humoral mediated cardiac allograft rejection. Ann Thorac Cardiovasc Surg. 2000;6:89-94.
- Bohnen MS, Iyer V, Sampson KJ, et al. Novel mechanism of transient outward potassium channel current regulation in the heart: implications for cardiac electrophysiology in health and disease. Circ Res 2015;116:1633-5. [Crossref]


