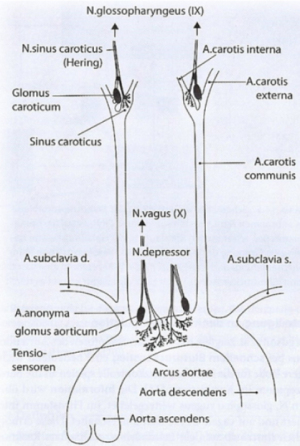
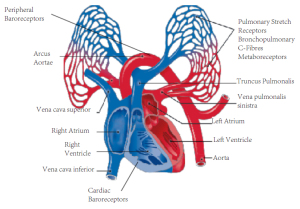
|
References
- Bigger JT Jr, Kleiger RE, Fleiss JL. Components of heart rate variability measured during healing of acute myocardial infarction. Am J Cardiol 1988;61:208-15.[LinkOut]
- Billman GE, Hoskins RS. Time-series analysis of heart rate variability during submaximal exercise: evidence for reduced cardiac vagal tone in animals susceptible to ventricular fibrillation. Circulation 1989;80:146-57.[LinkOut]
- Bilmann GE, Schwartz PJ, Stone HL. Baro-receptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation 1982;66:874-80.[LinkOut]
- Stein PK, Nelson P, Rottman JN, Howard D, Ward SM, Kleiger RE, et al. Heart rate variability reflects severity of COPD in PiZ α1-antitrypsin deficiency. Chest 1998;113:327-33.[LinkOut]
- Stewart AG, Waterhouse JC, Howard P. Cardiovascular autonomic nerve function in patients with hypoxaemic chronic obstructive pulmonary disease. Eur Respir J 1991;4:1207-14.[LinkOut]
- Volterrani M, Scalvini S, Mazzuero G. Decreased heart rate variability in patients with chronic obstructive pulmonary disease. Chest 1994;106:1432-7.[LinkOut]
- Steward RI, Lewis M. Cardiac output during exercise in patients with COPD. Chest 1986;89:199-205.[LinkOut]
- Patakas D, Louridas G, Kakavelas E. Reduced baroreceptor sensitivity in patients with chronic obstructive pulmonary disease. Thorax 1982;37:292-5.[LinkOut]
- Heindl S, Lehnert M, Criée CP. Marked sympathetic activation in patients with chronic respiratory failure. Am J Respir Crit Care Med 2001;164:597-601.[LinkOut]
- Velez-Roa S, Ciarka A, Najem B, Vachiery JL, Naeije R, van de Borne P. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation 2004;110:1308-12.[LinkOut]
- Poirier P, Lacasse Y, Marquis K, Jobin J, LeBlanc P. Post-exercise heart rate recovery and mortality in chronic obstructive pulmonary disease. Respiratory Medicine 2005;99:877-86.[LinkOut]
- Derchak PA, Sheel AW, Morgan BJ, Dempsey JA. Effects of expiratory muscle work on muscle sympathetic nerve activity. J Appl Physiol 2002;92:1539-52.[LinkOut]
- Hardy JC, Gray K, Whisler S, Leuenberger U. Sympathetic and blood pressure responses to voluntary apnea are augmented by hypoxemia. J Appl Physiol 1994;77:2360-5.[LinkOut]
- Leuenberger U, Gleeson K, Wroblewski K, Prophet S, Zelis R, Zwillich C, et al. Norepinephrine clearance is increased during acute hypoxemia in humans. Am J Physiol 1991;261:1659-64.[LinkOut]
- Saito M, Mano T, Abe H. Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol 1986;55:493-8.[LinkOut]
- Bartels M, Gonzalez J, Kim W, De Meersman R. Oxygen supplementation and cardiac-autonomic modulation in COPD. Chest 2000;118:691-6.[LinkOut]
- Scalvini S, Porta R, Zanelli E. Effects of oxygen on autonomic nervous system dysfunction in patients with chronic obstructive pulmonary disease. Eur Respir J 1999;13:119-24.[LinkOut]
- Bernardi L, Porta C, Spicuzza L. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002;105:143-5.[LinkOut]
- Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest 1991;87:1953-7.[LinkOut]
- Cooper VL, Pearson SB, Bowker CM, Elliott MW, Hainsworth R. Interaction of chemoreceptor and baroreceptor reflexes by hypoxia and hypercapnia - a mechanism for promoting hypertension in obstructive sleep apnoea. J Physiol 2005;568:677-87.[LinkOut]
- Raupach T, Bahr F, Herrmann P, LüthjeL, Hasenfuß G, Andreas S. Slow breathing reduces sympathoexcitation in COPD. Eur Respir J 2008;32:387-92.[LinkOut]
- Somers VK, Mark AL, Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension 1988;11;608-12.[LinkOut]
- Somers VK, Mark AL, Zavala DC. Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J Appl Physiol 1989;67:2095-100.[LinkOut]
- Somers VK, Mark AL, Abboud FM. Sympathetic activation by hypoxia and hypercapnia: implications for sleep apnea. Clin Exp Hypertens 1988;10:413-22.[LinkOut]
- Brown SJ, Howden R. The Effects of a Respiratory Acidosis on Human Heart Rate Variability. Adv Exp Med Biol 2008;605:361-65.[LinkOut]
- Sasano N, Vesely A, Hayano J, Sasano H, Somogyi R, Preiss D, et al. Direct effect of PaCO2 on respiratory sinus arrhythmia in conscious humans. Am J Physiol Heart Circ Physiol 2002;282:973-6.[LinkOut]
- Buda AJ, Pinsky MR, Ingles NB, Daugter GT, Stinson EB. Effect of intrathoracic pressure on left ventricular performance. N Engl J Med 1979:301:453-9.[LinkOut]
- Cournand A, Motley HL, Werko L, Richards DW. Physiological studies of the effects of intermittent positive pressure breathing on cardiac output in man. Am J Physiol 1948;152:162-74.[LinkOut]
- Calabrese P, Perrault H, Dinh TP, Eberhard A, Benchetrit G. Cardiovascular interactions during resistive load breathing. Am J Physiol Regul Intergr Comp Physiol 2000;279:2208-13.[LinkOut]
- Peters J, Kindred MK, Robotham JL. Transient analysis of cardiopulmonary interactions. I. Diastolic events. J Appl Physiol 1988;64:1506-17.[LinkOut]
- Ranieri VM, Dambrosio M, Brienza N. Intrinsic PEEP and cardiopulmonary interaction in patients with COPD and acute ventilatroy failure. Eur Respir J 1996;9:1283-92.[LinkOut]
- Pinsky MR. Cardiovascular Issues in respiratory care. Chest 2005;128:592-7.[LinkOut]
- Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol 2002;130:3-20.[LinkOut]
- Eckberg DL, Orshan CR. Respiratory and baroreceptor reflex interactions in man. J Clin Invest 1977;59:780-5.[LinkOut]
- Giardino ND, Chan L, Borson S. Combined heart rate variability and pulse oximetry biofeedback for chronic obstructive pulmonary disease: preliminary findings. Appl Psychophysiol Biofeedback 2004;29:121-33.[LinkOut]
- Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human R-R interval power spectra is largely ignored. J Appl Physiol 1993;75:2310-231.[LinkOut]
- Goso Y, Asanoi H, Ishise H. Respiratory modulation of muscle sympathetic nerve activity in patients with chronic heart failure. Circulation 2001;104:418-23.[LinkOut]
- Hirsch JA, Bishop B. Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. Am J Physiol Heart Circ Physiol 1981;241:620-9.[LinkOut]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 1985;57:461-9.[LinkOut]
- Mitchell JH, Schmidt RF. Cardiovascular reflex control by afferent fibers from skeletal muscle receptors. In: Shepherd JT, Abboud FM, editors. Handbook of physiology. The cardiovascular system. New York: Oxford University Press; 1983. p. 623-58.
- Dempsey JA, Sheel AW, St Croix CM. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol 2002;130:3-20.[LinkOut]
- Levine S, Nguyen T, Kaiser LR. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am J Respir Crit Care Med 2003;168:706-13.[LinkOut]
- Orozco-Levi M, Lloreta J, Minguella J. Injury of the human diaphragm associated with exertion and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1734-9.[LinkOut]
- Undem BJ, Kollarik M. The Role of Vagal Afferent Nerves in Chronic Obstructive Pulmonary Disease. Proc Am Thorac Soc 2005;2:355-60.[LinkOut]
- Tryphon S, Kontakiotis Th, Mavrofridis Eu, Patakas D. Hering-Breuer Reflex in Normal Adults and in COPD Patients. Respiration 2001;68:140-4.[LinkOut]
- Schelegle ES, Green JF. An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respiration Physiology 2001;125:17-31.[LinkOut]
- Gu Q, Lee LY. Alveolar hypercapnia augments pulmonary C-fiber responses to chemical stimulants: role of hydrogen ion. J Appl Physiol 2002;93:181-8.[LinkOut]
- Ma A, Bravo M, Kappagoda CT. Responses of bronchial C-fiber afferents of the rabbit to changes in lung compliance. Respir Physiol Neurobiol 2003;138:155-63.[LinkOut]
- Oudijk EJD, Lammers JWJ, Koenderman J. Systemic inflammation in chronic obstructive pulmonary disease. Eur Respir J 2003;22:5-13.[LinkOut]
- Jensen-Urstad M, Jensen-Urstad K, Ericson M, Johansson J. Heart rate variability is related to leucocyte count in men and to blood lipoproteins in women in a healthy population of 35-year-old subjects. J Intern Med 1998;243:33-40.[LinkOut]
- Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J 2004;25:363-70.[LinkOut]
- Anker SD, Coats AJ. Cardiac cachexia: a syndrome with impaired survival and immune and neuroendocrine activation. Chest 1999;115:836-47.[LinkOut]
- Andreas S, Anker SD, Paul D, Scanlon PD, Virend K, Somers VK. Neurohumoral activation as a link to systemic manifestations of chronic lung disease. Chest 2005;128;3618-24.[LinkOut]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405:458-62.[LinkOut]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003;421:384-8.[LinkOut]
- Bhattachaya J, Cunningham DJC, Howson MG. The effects of mild hypoxia with constant PACO2 and ventilation on the setting and sensitivity of the baroreceptor-cardiacdepressor reflex in man. J Physiol 1973;234:112-4.[LinkOut]
- Newton GE, Azevedo ER, Parker JD. Inotropic and sympathetic responses to the intracoronary infusion of a ß2-receptor agonist: a human in vivo study. Circulation 1999;99:2402-7.[LinkOut]
- Cekici L, Valipour A, Kohansal R, Burghuber OC. Short-term effects of inhaled salbutamol on autonomic cardiovascular control in healthy subjects: a placebo-controlled study. Br J Clin Pharmacol 2009l;67:394-402.[LinkOut]
- Silke B, Hanratty CG, Riddell JG. Heart-rate variability effects of beta-adrenoceptor agonists (xamoterol, prenalterol, and salbutamol) assessed nonlinearly with scatterplots and sequence methods. J Cardiovasc Pharmacol 1999;33:859-67.[LinkOut]
- Hanratty CG, Silke B, Riddell JG. Evaluation of the effect on heart rate variability of a beta2-adrenoceptor agonist and antagonist using non-linear scatterplot and sequence methods. Br J Clin Pharmacol 1999;47:157-66.[LinkOut]
- Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest 2004;125:2309-21.[LinkOut]
- Hardy JC, Gray K, Whisler S, Leuenberger U. Sympathetic and blood pressure responses to voluntary apnea are augmented by hypoxemia. J Appl Physiol 1994;77:2360-5.[LinkOut]
- Leuenberger U, Gleeson K, Wroblewski K, Prophet S, Zelis R, Zwillich C, et al. Norepinephrine clearance is increased during acute hypoxemia in humans. Am J Physiol 1991;261:1659-64.[LinkOut]
- Bartels MN, Jelic S, Ngai P, Basner RC, DeMeersman RE. High-frequency modulation of heart rate variability during exercise in patients with COPD. Chest 2003;124:863-9.[LinkOut]
- O’Donell DE, Lavenesiana P. Physiology and consequences of lung hyperinflation in COPD. Eur Respir Rev 2006;100:61-7.[LinkOut]
- Engström G, Gerhardsson de Verdier M, Dahlbäck M, Janson C, Lars Lind L. BP variability and cardiovascular autonomic function in relation to forced expiratory volume: a population-based study. Chest 2009;136:177-83.[LinkOut]
- Bauerle O, Chrusch CA, Younes M. Mechanisms by which COPD affects exercise tolerance. Am J Respir Crit Care Med 1998;157:57-68.[LinkOut]
- West JB, Wagner PD, Neder JA, Scano GL, Jones NL, Zakynthinos SG, et al. The major limitation to exercise performance in COPD is inadequate energy supply to the respiratory and locomotor muscles vs. lower limb muscle dysfunction vs. dynamic hyperinflation. J Appl Physiol 2008;105:758-62.[LinkOut]
- Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation 1997;96:526-34.[LinkOut]
Cite this article as: van Gestel AJR, Steier J. Autonomic dysfunction in patients with chronic obstructive pulmonary disease (COPD). J Thorac Dis 2010;2(4):215-222. doi: 10.3978/j.issn.2072-1439.2010.02.04.5
|