Localization of thoracic duct using heavily T2W MRI for intractable post-esophagectomy chylothorax—a case report
Introduction
Esophageal squamous cell carcinoma (ESCC) is a malignant tumor with high morbidity and mortality. The standard treatment for resectable ESCC is esophagectomy with adequate lymphadenectomy (1). In recent years, chemotherapy and radiotherapy, in addition to surgery, has been shown to be effective for locally advanced ESCC (2).
Thoracoscopic esophagectomy is currently accepted as the procedure of choice by a large number of surgeons due to minimal trauma and rapid recovery (3). However, esophagectomy still is a technically challenging operation with considerable potential for various postoperative complications. Among these complications, chylothorax is a rare but a serious complication that is associated with potentially life-threatening respiratory, metabolic and immunologic morbidity (4). We herein presented the case of a patient with ESCC who underwent thoracoscopic esophagectomy that was complicated with persistent and intractable post-esophagectomy chylothorax. The remnant duct was localized with T2-weighted magnetic resonance imaging (T2W MRI), followed by thoracoscopic thoracic duct ligation. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Case presentation
A 51-year-old male with no significant medical history was diagnosed with multi-focal moderately to well-differentiated ESCC, clinical stage T1N1M0G1Lu (according to American Joint Committee on Cancer staging, 7th edition). He underwent neo-adjuvant chemo-radiotherapy with a regimen of cisplatin plus fluorouracil every week for seven cycles, and total dosage of 3,600 cGy of radiation therapy. This was followed by video-assisted thoracoscopic esophagectomy with mediastinal lymph nodes dissection in left lateral decubitus position; midline laparotomy gastric tube formation with bilateral modified neck lymph node dissection, and reconstruction via a posterior mediastinal route with esophagogastrostomy at the neck level in supine position. After dissecting the esophagus and mediastinal lymph nodes, the thoracic duct was prophylactically mass ligated above the diaphragmatic hiatus at the level of the tenth intercostal space by performing surgical ties with non-absorbable silk after encircling all the lympho-fatty tissues between the aorta and the azygos vein. The above procedure was performed without any attempt to identify the thoracic duct. There was also no remarkable damage of the thoracic duct during the operation, and the final pathological diagnosis revealed no residual tumor or lymph node metastasis.
Left side tube thoracostomy was performed due to massive left pleural effusion occurred postoperatively, a large amount of bilateral chest tube drainage was noted. The triglyceride level of the right chest tube drainage was 230 mg/dL on postoperative day five. Conservative treatment including total parenteral nutrition and adequate drainage was attempted, but the chyle leak was uncontrollable, and continued at over 1,000 mL/day (Figure 1). Due to a suspicious existence of aberrant collaterals of thoracic ducts despite performing thoracic duct mass ligation at first operation, bilateral video-assisted thoracoscopic thoracic duct mass ligation both above the level of diaphragm with chemical and mechanical pleurodesis and bilateral decortications was performed on postoperative day six. The position and level of lympho-fatty tissues were encircled and mass ligated lower than the first operation just above the diaphragm in the right thoracic cavity and between aorta and thoracic spine in the left thoracic cavity separately. However, the chyle leak persisted after the second operation despite an intravenous infusion of somatostatin (6 mg/day) beginning postoperative day seven.
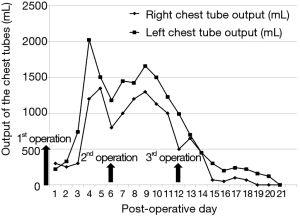
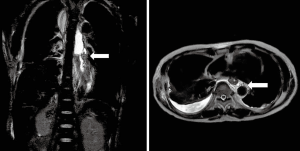
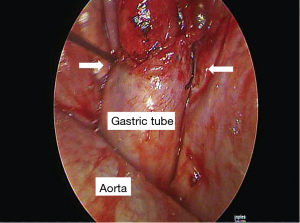
Heavily T2W MRI (respiratory-triggered three-dimensional T2-weighted magnetic resonance cholangiopancreatography on a 1.5-T MR scanner, Philips Achieva system), as used in magnetic resonance cholangiopancreatography (MRCP) was performed for localization of the remnant lymphatic duct. The imaging revealed a dilated lymphatic duct between the gastric tube and aorta at the T7 to T9 level (Figure 2). Thoracic duct embolization was attempted by an interventional radiologist using computed tomography and fluoroscopic guidance on post-operative day 12, but the procedure was eventually abandoned because of repeated puncture failure. Repeat video-assisted thoracoscopic lymphatic duct ligation at the T7-9 level between the gastric tube and aorta was performed the same day as the MRI based on the MRI findings (Figure 3). Postoperatively, the amount of chest drainage decreased bilaterally, and the chest tubes were removed on postoperative day 10. The patient had no problems beginning oral intake, and was discharged on postoperative day 43. The patient was alive and well at 6 months postoperatively.
Discussion
Chylothorax can occur after any type of thoracic operation. With esophagectomy, chylothorax most often occurs due to thoracic duct injury during mediastinal dissection (5). The overall incidence of developing a chylothorax after esophagectomy has been reported to range from 2–4% in a high-volume center (6,7). Persistent chyle loss, and its accumulation within the pleural cavity can have serious local, metabolic, and immunological effects which may have a significant impact on postoperative outcome, such as higher instances of pneumonia and arrhythmia, and a longer hospital stay after esophagectomy (8-10). Currently, chylothorax remains a challenging problem, and no single method for its treatment has been proven to be effective. Mortality rates as high as 50% have been reported (11). Thus, improving the management of chylothorax further is an issue that needs to be addressed.
A systemic review and institutional analysis by Kranzfelder et al. indicated that postoperative chylothorax occurred significantly more frequently among patients with SCC (squamous cell carcinoma) than among patients with adenocarcinoma (AC) of the esophagus, and significantly more frequently among patients who had surgery after neoadjuvant treatment than among patients undergoing surgery without any neoadjuvant treatment (7). A study by Merritt et al. also reported a higher incidence of chylothorax in patients who received neoadjuvant therapy as compared to those who received esophagectomy alone (12). In an analysis of perioperative factors, Gupta et al. found that patients with chylothorax had tumors in the middle third of the thoracic esophagus, and the usual site of injury to the thoracic duct was in the mid-thorax during mediastinal dissection (13). A similar finding was reported by Rao et al. who found that the incidence of chylothorax in middle-third lesions was relatively higher than that in lower-third lesions (14). Thus, it is believed that the thoracic duct is in close proximity to the middle third of the thoracic esophagus, thus making it prone to injury during mediastinal dissection despite the surgeon’s vigilance. Another risk factor for developing a post-esophagectomy chylothorax is a body mass index (BMI) <25 kg/m2 (15), or BMI <30 kg/m2 (16). Our patient had several risk factors for developing post-esophagectomy chylothorax, including a BMI of 20 kg/m2 before esophagectomy, ESCC in middle third of the thoracic esophagus, and receiving neoadjuvant chemo-radiotherapy.
Prophylactic thoracic duct mass ligation has been advocated for preventing or minimized the risk of post-esophagectomy chylothorax, even for patient treated with preoperative chemo-radiotherapy (17). The results of a randomized controlled trial (RCT) by Lai et al. indicated that the incidence of postoperative chylothorax was significantly lower in patients who receive prophylactic thoracic duct ligation (18). Since ligation of the thoracic duct is successful in 90% of patients when performed just above the right hemi-diaphragm (19), a site that has the advantage of halting flow from any unidentified accessory ducts, we routinely perform prophylactic thoracic duct mass ligation at the aforementioned site after dissecting the esophagus. However, although video-assisted prophylactic thoracic duct ligation is an effective approach and can minimize the risk of postoperative chylothorax, the incidence of chylothorax after preventive ligation remains at about 2.7% (8,15). The anatomic variation such as duplicate thoracic duct observed in 39–47% of patients and collaterals of lymph ducts may be the cause (8).
Postoperative chylothorax typically becomes clinically apparent 2–7 days after surgery. Chylothorax is typically diagnosed by a change in the quality of chest tube drainage to a milky white drainage, regardless of the drainage amount. However, because the drainage has the classic milky white appearance in only 50% of cases (20), the exact diagnosis is based on the present of chylomicrons in a patient with large amount of chest tube drainage, or a drainage triglyceride level >1.24 mmol/L (110 mg/dL) with a cholesterol level <5.18 mmol/L (200 mg/dL) (21).
Treatment of post-esophagectomy chylothorax can involve both conservative and surgical methods. The initial management is often conservative, and includes drainage tube insertion, total parenteral nutrition, and the administration of somatostatin/octreotide. If there is no improvement after conservative therapy, surgical intervention by video-assisted thoracic duct mass ligation should be performed. In addition, percutaneous embolization of the thoracic duct using interventional radiological techniques has recently been introduced as a minimally invasive and less risky method, which has been advocated to treat any cause of chylothorax (10,22). However, the procedure is technically challenging, and the success rates vary widely between different centers, with an overall success rate of about 70% (23). On the other hand, the reported success rate of surgical thoracic duct ligation is higher than 90% (7,19). Since surgical reoperation is thought to be the most reliable therapeutic method for a severe chyle leak, it is generally advised that conservative treatment should not be attempted for more than 2 weeks (24). However, Kranzfelder et al. reported that reoperation should be performed if a chyle leak persists for more than 1 week (rather than 2 weeks), or pleural drainage is greater than 1 liter per day for more than 5 days (7). In addition, Miao et al. have advocated that surgery should be performed in patients who develop chylothorax early after surgery (before the fourth postoperative day), or if the chyle output is ≥13.5 mL/kg after 3 days of conservative therapy (15). Although there is no generally accepted consensus on the indication and timing of surgical intervention, it has been reported that post-esophagectomy chylothorax managed with re-exploration is associated with a mortality of 10% compared to a mortality of 50% if conservative management is used as the mainstay of treatment (11,16). Thus, some authors recommend early surgical treatment to decrease the risk of mortality.
The main problem of open procedures is that the exact location of the leak cannot always be identified, which results in a decreased success rate. Although lymphoscintigraphy is a non-invasive method for detecting the cisterna chyli, conventionally bipedal lymphangiography is performed in very few hospitals because of the contrast agent effect, the long time of examination required and a lower detection rate for the cisterna chyli of about 53% (25). In recent years, the sensitivity of MRI has proven to be superior to computed tomography (CT) in imaging lymphatic channels (26). In addition, a method for thoracic duct visualization using three-dimensional T2WI has been described (27). Another report has indicated that non-contrast-enhanced MRI is feasible for the detection of morphological changes of thoracic lymphatics, and in the identification of chyloma and leak sites in patients with chylothorax (28). Furthermore, a technique of using T2W MRI for guiding thoracic duct puncture and direct injection of glue through the puncture needle under fluoroscopic guidance without cannulating the duct, instead of using bipedal lymphangiography, has been published (29).
In our case, since a right-side thoracoscopic approach had been done at the first ligation and since we suspected that it might exist an aberrant collaterals of thoracic ducts along the lympho-fatty tissues between aorta and thoracic spine in the left thoracic cavity or between the aorta and the azygos vein in the right thoracic cavity, bilateral thoracoscopic procedures was performed at second ligation surgery. Unexpectedly, T2W MRI revealed that the patient had a rare anatomical variation of the thoracic ducts: a rare anatomical variation of a dilated lymphatic duct between the gastric tube and aorta at the T7–9 level, whereas we mass ligated the lympho-fatty tissues between aorta and thoracic spine during the first left side thoracic duct mass ligation at second ligation surgery. Repeated thoracic duct mass ligation at the T8 level between the gastric tube and aorta was performed based on the MRI findings.
In conclusion, we described a patient with intractable post-esophagectomy chylothorax despite having received prophylactic thoracic duct mass ligation, conservative treatment, and repeated thoracoscopic thoracic duct mass ligation. The chyle leak was successfully stopped by a third thoracoscopic thoracic duct mass ligation using T2W MRI for localization of the dilated lymphatic duct.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Low DE. Update on staging and surgical treatment options for esophageal cancer. J Gastrointest Surg 2011;15:719-29. [Crossref] [PubMed]
- Kranzfelder M, Schuster T, Geinitz H, et al. Meta-analysis of neoadjuvant treatment modalities and definitive non-surgical therapy for oesophageal squamous cell cancer. Br J Surg 2011;98:768-83. [Crossref] [PubMed]
- Nguyen NT, Follette DM, Wolfe BM, et al. Comparison of minimally invasive esophagectomy with transthoracic and transhiatal esophagectomy. Arch Surg 2000;135:920-5. [Crossref] [PubMed]
- Baba Y, Yoshida N, Shigaki H, et al. Prognostic Impact of Postoperative Complications in 502 Patients With Surgically Resected Esophageal Squamous Cell Carcinoma: A Retrospective Single-institution Study. Ann Surg 2016;264:305-11. [Crossref] [PubMed]
- Mishra PK, Saluja SS, Ramaswamy D, et al. Thoracic duct injury following esophagectomy in carcinoma of the esophagus: ligation by the abdominal approach. World J Surg 2013;37:141-6. [Crossref] [PubMed]
- Alexiou C, Watson M, Beggs D, et al. Chylothorax following oesophagogastrectomy for malignant disease. Eur J Cardiothorac Surg 1998;14:460-6. [Crossref] [PubMed]
- Kranzfelder M, Gertler R, Hapfelmeier A, et al. Chylothorax after esophagectomy for cancer: impact of the surgical approach and neoadjuvant treatment: systematic review and institutional analysis. Surg Endosc 2013;27:3530-8. [Crossref] [PubMed]
- Merigliano S, Molena D, Ruol A, et al. Chylothorax complicating esophagectomy for cancer: a plea for early thoracic duct ligation. J Thorac Cardiovasc Surg 2000;119:453-7. [Crossref] [PubMed]
- Hematti H, Mehran RJ. Anatomy of the thoracic duct. Thorac Surg Clin 2011;21:229-38. ix. [Crossref] [PubMed]
- Cope C, Salem R, Kaiser LR. Management of chylothorax by percutaneous catheterization and embolization of the thoracic duct: prospective trial. J Vasc Interv Radiol 1999;10:1248-54. [Crossref] [PubMed]
- Bolger C, Walsh TN, Tanner WA, et al. Chylothorax after oesophagectomy. Br J Surg 1991;78:587-8. [Crossref] [PubMed]
- Merritt RE, Whyte RI, D'Arcy NT, et al. Morbidity and mortality after esophagectomy following neoadjuvant chemoradiation. Ann Thorac Surg 2011;92:2034-40. [Crossref] [PubMed]
- Gupta R, Singh H, Kalia S, et al. Chylothorax after esophagectomy for esophageal cancer: risk factors and management. Indian J Gastroenterol 2015;34:240-4. [Crossref] [PubMed]
- Rao DV, Chava SP, Sahni P, et al. Thoracic duct injury during esophagectomy: 20 years experience at a tertiary care center in a developing country. Dis Esophagus 2004;17:141-5. [Crossref] [PubMed]
- Miao L, Zhang Y, Hu H, et al. Incidence and management of chylothorax after esophagectomy. Thorac Cancer 2015;6:354-8. [Crossref] [PubMed]
- Shah RD, Luketich JD, Schuchert MJ, et al. Postesophagectomy chylothorax: incidence, risk factors, and outcomes. Ann Thorac Surg 2012;93:897-903; discussion 903-4. [Crossref] [PubMed]
- Cagol M, Ruol A, Castoro C, et al. Prophylactic thoracic duct mass ligation prevents chylothorax after transthoracic esophagectomy for cancer. World J Surg 2009;33:1684-6. [Crossref] [PubMed]
- Lai FC, Chen L, Tu YR, et al. Prevention of chylothorax complicating extensive esophageal resection by mass ligation of thoracic duct: a random control study. Ann Thorac Surg 2011;91:1770-4. [Crossref] [PubMed]
- Paes ML, Powell H. Chylothorax: an update. Br J Hosp Med 1994;51:482-90. [PubMed]
- Rahman NM, Chapman SJ, Davies RJ. Pleural effusion: a structured approach to care. Br Med Bull 2005;72:31-47. [Crossref] [PubMed]
- Staats BA, Ellefson RD, Budahn LL, et al. The lipoprotein profile of chylous and nonchylous pleural effusions. Mayo Clin Proc 1980;55:700-4. [PubMed]
- Cope C. Diagnosis and treatment of postoperative chyle leakage via percutaneous transabdominal catheterization of the cisterna chyli: a preliminary study. J Vasc Interv Radiol 1998;9:727-34. [Crossref] [PubMed]
- Atie M, Dunn G, Falk GL. Chlyous leak after radical oesophagectomy: Thoracic duct lymphangiography and embolisation (TDE)-A case report. Int J Surg Case Rep 2016;23:12-6. [Crossref] [PubMed]
- Teba L, Dedhia HV, Bowen R, et al. Chylothorax review. Crit Care Med 1985;13:49-52. [Crossref] [PubMed]
- Verma SK, Mitchell DG, Bergin D, et al. Dilated cisternae chyli: a sign of uncompensated cirrhosis at MR imaging. Abdom Imaging 2009;34:211-6. [Crossref] [PubMed]
- Erden A, Fitoz S, Yagmurlu B, et al. Abdominal confluence of lymph trunks: detectability and morphology on heavily T2-weighted images. AJR Am J Roentgenol 2005;184:35-40. [Crossref] [PubMed]
- Yu DX, Ma XX, Zhang XM, et al. Morphological features and clinical feasibility of thoracic duct: detection with nonenhanced magnetic resonance imaging at 3.0 T. J Magn Reson Imaging 2010;32:94-100. [Crossref] [PubMed]
- Yu DX, Ma XX, Wang Q, et al. Morphological changes of the thoracic duct and accessory lymphatic channels in patients with chylothorax: detection with unenhanced magnetic resonance imaging. Eur Radiol 2013;23:702-11. [Crossref] [PubMed]
- Praveen A, Sreekumar KP, Nazar PK, et al. Technical Note: Thoracic duct embolization for treatment of chylothorax: A novel guidance technique for puncture using combined MRI and fluoroscopy. Indian J Radiol Imaging 2012;22:89-92. [Crossref] [PubMed]


