Variations in positron emission tomography-computed tomography findings for patients receiving neoadjuvant and non-neoadjuvant therapy for non-small cell lung cancer
Introduction
Precise evaluation of cancer stage is needed before cancer treatment, especially for surgery planning. In spite of advances in endobronchial ultrasound (EBUS), endoscopic ultrasound (EUS), and mediastinoscopic biopsy, Positron emission tomography-computed tomography (PET-CT) is widely accepted as a standard tool for noninvasive evaluation of cancer status, especially distant or loco-regional lymph node (LN) metastases in non-small cell lung cancer (NSCLC) (1-5). Because cancer cells have increased number of glucose transporters and are highly metabolically active, the radiopharmaceutical 18fludeoxyglucose (18FDG) is a glucose analog used to evaluate tumor metabolism (6). Correlations between the maximum standard uptake value (SUVmax) and cancer progression are established (6-8).
Neoadjuvant therapy is usually recommended for NSCLC with N2 disease status by pathological confirmation, if clinically feasible (7,9-11). A lesion is usually regarded to be malignant or metastatic when SUVmax is 2.5 or higher and the lesion diameter is larger than 1cm on PET-CT (3,12,13). However, discrepancies between pathological and preoperative PET-CT findings are common, especially for LNs (7). Thus preoperative PET-CT findings might provide incorrect information and influence management plan for NSCLC (1,6,14). In addition, whether PET-CT findings differ between patients treated with neoadjuvant therapy (NT) or without neoadjuvant therapy (non-NT) for NSCLC remains unclear (7,11,15). The influences of neoadjuvant therapy on PET-CT findings in NSCLC are unknown (7,9,15). The aims of this study were to predict LN metastases using preoperative PET-CT findings and investigate differences in PET-CT findings between NT and non-NT for NSCLC.
Methods
Patients and methods
Data from 578 consecutive patients who underwent surgery for pathologically proven NSCLC at a single tertiary general hospital from January 2010 to December 2015 were retrospectively assessed and included. Inclusion criteria were anatomical and complete resection (R0) with standard mediastinal lymph node dissection, curative intent cases, histology of NSCLC, and PET-CT acquisition for initial evaluation before treatment and re-evaluation after neoadjuvant therapy. Exclusion criteria were postoperative death within one month due to postoperative complications, concurrent other primary cancer cases, palliation or salvage treatment, and concurrent active inflammation such as pneumonia. Preoperative evaluations were chest CT, PET-CT, bone scan, brain magnetic resonance imaging (MRI), EBUS, and mediastinoscopy if needed. Neoadjuvant and adjuvant therapies were conducted in accordance with the National Comprehensive Cancer Network guidelines and suggestions of a multidisciplinary team who reviewed cancer status, resectability or operability, and patient conditions. Neoadjuvant therapy was usually two cycles of cisplatin and paclitaxel, with radiation therapy over five weeks for a total of 44–45 Gray. Re-evaluation by PET-CT was conducted 4 weeks after neoadjuvant therapy completion, and further management plan was determined. Surgery was usually preformed five or six weeks after neoadjuvant therapy completion in NT. Patients were followed at 3-month intervals one year after treatment completion including adjuvant therapy and then at 6-month intervals by chest CT. Recurrence or metastasis was diagnosed based on imaging findings including PET-CT, brain MRI, and bone scan, or pathological confirmation when clinically feasible. Preoperative stage for NT was defined as clinical stage by PET-CT re-evaluation after neoadjuvant therapy and before surgery. Mediastinoscopy or EBUS biopsy was employed for LN SUVmax ≥2.5 and the size was ≥1 cm on PET-CT scan (2). Surgeries were performed by three thoracic surgeons using video-assisted thoracoscopic surgery or conventional thoracotomy with standard mediastinal lymph node dissection depending on cancer status and patient condition. PET-CT SUVmax data on tumors and LNs (divided into N1 and N2 LN) were retrospectively reviewed. To investigate variations in PET-CT findings between NT and non-NT and predict LN metastases, we analyzed correlations between SUVmax and pathological stage, compared disease-free survival (DFS) and overall survival (OS), investigated the associations between tumor and LN SUVmax values, evaluated and predicted LN metastases using SUVmax values, compared pathologically negative and positive LNs using SUVmax values, and assessed influences of neoadjuvant therapy on SUVmax. All LNs were analyzed separately for N1 and N2. Histopathological findings of specimens were analyzed using immunohistochemistry staining of single (for LN) and multiple (for tumor) sections and all slides were independently evaluated by two expert pathologists. Cancer stage was determined in accordance with the seventh American Joint Committee on Cancer staging system.
Procedures for PET-CT examinations
Written informed consent was given by all patients before PET-CT (Siemens Healthcare, Erlangen, Germany) examinations for potential future use of their clinical data for research. Patients fasted for more than six hours before examinations, and blood glucose levels were checked before intravenous injection of 18FDG. Examinations were deferred for blood glucose >160 mg/dL. Positron emission images were acquired from skull base to mid-thigh level one or two hours after injection of 18FDG (total dose, 60–80 mCi). CT scans were concurrently acquired with positron emission scans for exact anatomic localization of hypermetabolic lesions. SUV were calculated by the software for hypermetabolic regions detected on images. SUVmax was calculated by identifying a region of interest in an axial slice with the highest uptake of 18FDG within a lesion and was used to determine 18FDG uptake within the lesion. Two nuclear medicine specialists independently assessed SUVmax values for all scans. Medical records were referred to distinguish malignancy or metastasis from nonspecific lesions. We compiled SUVmax values as the highest uptake of 18FDG within tumors and individual LNs that were later pathologically dissected and confirmed. LN SUVmax was defined as the highest SUVmax value among all pathologically dissected and confirmed LNs. LNs with SUVmax ≥2.5 and size ≥1 cm were regarded as positive for metastasis, in accordance with previous studies and our hospital policy (8,16).
Statistical considerations and study approval
Comparisons between subgroups were evaluated using the Student T or Paired T test. Categorical variables were compared with the chi-square or the Fisher’s exact tests. The Pearson test was used to evaluate associations between continuous variables, and the Kaplan-Meier survival estimations were conducted with the log-rank tests used to test differences in survival across strata. Propensity score matching method was used to compare NT and non-NT to overcome heterogeneity. The receiver operating characteristic (ROC) analysis was conducted for diagnostic evaluations. The Statistical Package of Social Sciences version 22.0 (SPSS, Chicago, IL, USA) was used for all analyses. A P value <0.05 was regarded as statistically significant. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (IRB Approval number: KC16RIS10839).
Results
Patient population
Consecutive 578 patients (male 315, female 263; mean age 63.6±9.5 years) who underwent curative and complete surgery for NSCLC from January 2010 to December 2015 were included. Preoperative pathological confirmation of LN using EBUS or mediastinoscopy was conducted for 71.5%. A total of 66 patients received neoadjuvant therapy (initial clinical stage before neoadjuvant therapy: IIa 4, IIb 1, IIIa 50, and IIIb 11 cases). All histology results showed NSCLC. Surgery methods were segmentectomy (19 cases), lobectomy (540 cases), bilobectomy (16 cases) and pneumonectomy (3 cases). Mean tumor size was 8.1±11.7 cm2. Mean SUVmax was 5.9 (±4.7) for tumor, 2.3 (±2.0) for N1 LN, and 2.2 (±1.9) for N2 LN. Mean number of LNs dissected was 14.2 (±8.0). Mean observation period was 30.6 (±21.4) months. Overall clinico-pathological characteristics for patient population are in Table 1.
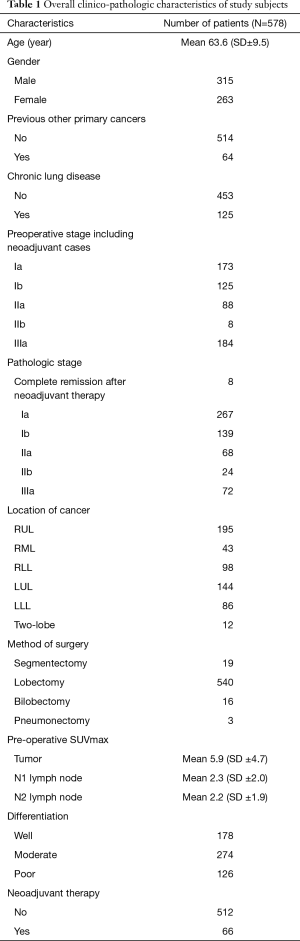
Full table
Associations between SUVmax and cancer progression
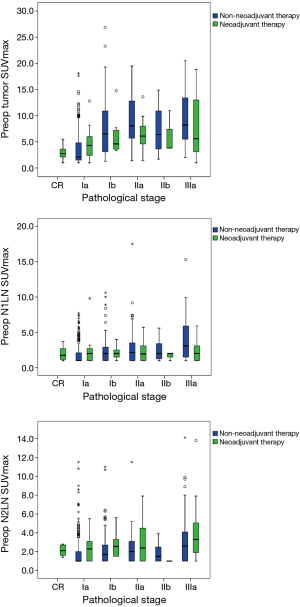
For non-NT, we found significantly positive associations between pathological stage and SUVmax (tumor, N1 LN, and N2 LN, all P<0.001). For NT, we found positive associations between pathological stage and tumor and N2 LN SUVmax, but not N1 LN (tumor P=0.005, N1 LN P=0.981, N2 LN P=0.045) (Figure 1). For non-NT, tumor and LN SUVmax were higher with lymphovascular invasion compared to without (tumor and N1 LN SUVmax P<0.001, N2 LN SUVmax P=0.001). However, for NT, tumor and LN SUVmax were not different with or without lymphovascular invasion.
DFS and OS analyses according to SUVmax
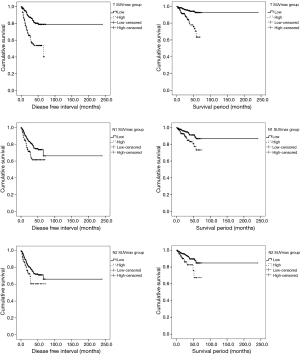
We divided patients into two groups (low and high) by mean preoperative SUVmax (non-NT: tumor SUVmax 5.9, N1 LN SUVmax 2.3, N2 LN SUVmax 2.1; NT: tumor SUVmax 5.9, N1 LN SUVmax 2.2, and N2 LN SUVmax 3.0). For non-NT, the low SUVmax group had higher DFS and OS than the high SUVmax group (DFS: tumor SUVmax P<0.001, N1 LN SUVmax P=0.002, N2 LN SUVmax P=0.027; OS: tumor SUVmax P<0.001, N1 LN SUVmax P=0.006, N2 LN SUVmax P=0.006) (Figure 2). For NT, the low SUVmax group had higher DFS and OS than the high SUVmax group; however, these findings were not significant (DFS: tumor SUVmax P=0.227, N1 LN SUVmax P=0.693, N2 LN SUVmax P=0.051; OS: tumor SUVmax P=0.621, N1 LN SUVmax P=0.542, N2 LN SUVmax P=0.115). In addition, to investigate survival according to the degree of SUVmax changes after neoadjuvant therapy, we divided NT into the high and the low group by the mean value of the degree of SUVmax changes (N1LN SUVmax 2.0 and N2LN SUVmax 2.9). We found no significant differences in survival between the high and low group (DFS: N1 LN P=0.839, N2 LN P=0.504; OS: N1 LN P=0.342, N2 LN P=0.860).
Associations between tumor and LN SUVmax
For non-NT, tumor, N1 LN, and N2 SUVmax were positively correlated (all P<0.001). For NT, tumor and N2 SUVmax (P<0.001), and N1 and N2 SUVmax (P=0.025) were positively associated, but not tumor and N1 LN SUVmax (P=0.911).
Receiver operating characteristic analysis for predicting LN metastasis and complete remission using SUVmax
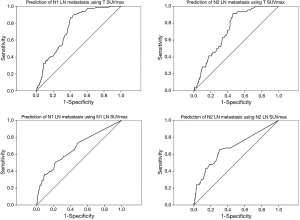
For non-NT, 38 of 341 patients with N1 LN SUVmax <2.5 had pathologically positive N1 LN and 22 of 366 patients with N2 LN SUVmax <2.5 had pathologically positive N2 LN. In addition, 126 of 171 patients with N1 LN SUVmax ≥2.5 had pathologically negative N1 LN, and 122 of 146 patients with N2 LN SUVmax ≥2.5 had pathologically negative N2 LN. The ratio of LN (N1 and N2) SUVmax to tumor SUVmax was not associated with LN positivity. For NT, 18 of 35 patients with N1 LN SUVmax <2.5 had pathologically positive N1 LN and 7 of 27 patients with N2 LN SUVmax <2.5 had pathologically positive N2 LN. In addition, 11 of 17 patients with N1 LN SUVmax ≥2.5 had pathologically negative N1 LN, and 16 of 25 patients with N2 LN SUVmax ≥2.5 had pathologically negative N2 LN. For non-NT, ROC analysis revealed cutoff values for tumor and LN SUVmax for significant prediction of LN metastases (N1 LN: tumor SUVmax cutoff 5.95 sensitivity 66.3%, specificity 66.0%, area =0.748, P<0.001; N1 LN SUVmax cutoff 2.05, sensitivity 57.83%, specificity 66.43%, area =0.676, P<0.001) (N2 LN: tumor SUVmax cutoff 5.95, sensitivity 63.04%, specificity 63.95%, area =0.726, P<0.001; N2 LN SUVmax cutoff 2.05, sensitivity 65.22%, specificity 69.96%, area =0.678, P<0.001). However, for NT, ROC analysis showed no significant findings for predicting LN metastases using SUVmax values. ROC analysis showed no significant findings for predicting complete remission using tumor and LN SUVmax. ROC analysis using SUVmax to evaluate LNs metastasis is shown in Figure 3. To predict LN metastases for better diagnostic accuracy and prognosis, for NT we investigated new findings for NT using primary tumor SUVmax. If tumor SUVmax was higher than 5.9 (mean of tumor SUVmax for NT), N2 LN metastasis could be predicted using tumor SUVmax (cutoff for tumor SUVmax was 8.1, area 0.757, sensitivity 87.5%, and specificity 61.1%, P=0.040). However, predicting N1 LN was difficult. When primary tumor SUVmax was lower than the mean of the tumor SUVmax, Predicting N1 and N2 LN metastasis was difficult.
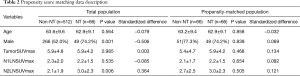
Comparison of NT and non-NT after the propensity score matching
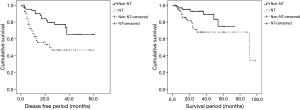
The propensity score matching was used to overcome heterogeneities in data between NT and non-NT (Table 2). When age, sex, and SUVmax were equal, NT pathological stage were significantly higher than non-NT stages (P<0.001). Non-NT had significantly higher DFS (P=0.001) and OS (P=0.024) than NT (Figure 4).
Full table
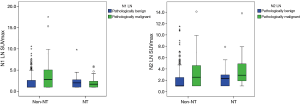
Neoadjuvant therapy effects on tumor and LN SUVmax
A total of 66 patients received neoadjuvant therapy (initial clinical stage before neoadjuvant therapy: IIa, 4; IIb, 1; IIIa, 50; IIIb, 11). After neoadjuvant therapy, clinical stage determined via PET-CT changed with upstaging for 1 of 66 (1.5%), downstaging for 32 of 66 (48.5%) and no change for 33 of 66 (50%). Decreases in tumor and N1 and N2 LN SUVmax were significant (all P<0.001). We found significant decreases in SUVmax in both pathologically malignant and benign LN after neoadjuvant therapy (malignant LN: N1 LN P=0.001, N2 LN P=0.024; benign LN: N1 LN P=0.004 LN N2 P<0.001). However, we found no significant difference in the degree of SUVmax decrease between pathologically malignant and benign LN (N1 LN P=0.988, N2 LN P=0.963). We found no significant differences in LN SUVmax between pathologically malignant and benign LN (N1 LN P=0.570, N2 LN P=0.105) after neoadjuvant therapy. However, for non-NT, LN was significantly higher for pathologically malignant LN than pathologically benign LN (N1 LN P<0.001, N2 LN P=0.001) (Figure 5). After neoadjuvant therapy, tumor and N2 LN SUVmax for patients with non-complete remission (non-CR) were significantly higher than for patients in complete remission (CR), except for those with N1 LN SUVmax (tumor P<0.001, N1 LN P=0.880, N2 LN P=0.026). We found significant decreases in tumor and LN SUVmax in both CR and non-CR after neoadjuvant therapy (CR: tumor P<0.001, N1 LN P=0.150, N2LN P=0.079; non-CR: all P<0.001).
Discussion
PET-CT is a standard non-invasive modality for assessing cancer status, especially for distant or LN metastasis of NSCLC (6). When N2 disease is suspected using PET-CT, neoadjuvant therapy instead of prompt surgery is considered as an initial treatment after pathological confirmation, if clinically feasible (7,9,10). Thus, PET-CT contributes to appropriate management of NSCLC by providing of qualitative and quantitative information from measuring lesion metabolic activity (1,12,14). However, discrepancies between PET-CT and pathological findings are frequent, especially for LN (4,15). In addition, the lack of established features or qualitative PET-CT findings results in variation among institutes in evaluation of LN status in NSCLC. To clarify variations in PET-CT findings in NSCLC, findings from PET-CT for NT and non-NT were investigated, especially for predicting LN metastasis using PET-CT, from thoracic surgeons’ perspective.
This study showed that tumor and LN SUVmax were significantly correlated with NSCLC progression by pathological stage, regardless of neoadjuvant therapy, except for N1 LN SUVmax for NT. In addition, tumor and LN SUVmax were significantly correlated with survival (DFS and OS) only for non-NT.
For non-NT, ROC analysis showed PET-CT might predict N1 and N2 LN status (both N1 and N2). However, for NT, findings for predicting LN metastases using SUVmax were not significant. In addition, prediction of complete remission using tumor and LN SUVmax was not possible. A significant decrease in SUVmax value was found for both pathologically malignant and benign LN after neoadjuvant therapy. Tumor and LN SUVmax decreases in both CR and non-CR. The inability to distinguish CR from non-CR using the SUVmax value was assumed to be caused by the considerable portion of LNs that were pathologically malignant converting to benign after neoadjuvant therapy, and less effect of neoadjuvant therapy develops in pathologically malignant LNs than in pathologically benign ones. These results indicated that distinguishing pathologically malignant and benign LN using only SUVmax values after neoadjuvant therapy was not possible. In addition, SUVmax was not an appropriate diagnostic value for predicting LN metastasis for NT, indicating the importance of considering other conditions and pathological confirmation to assess LNs, if clinically feasible. Several other primary cancers studies have shown the possibility of predicting LN metastasis after neoadjuvant therapy based on SUVmax of the primary lesion (13,15). However, the possibility of predicting LN using the primary lesion SUVmax after neoadjuvant therapy was not possible in our study. Prediction of LN metastases based on SUVmax of primary lesions was possible only for non-NT, probably due to the heterogeneous effects of neoadjuvant therapy on tumor and LN. Therefore, to predict LN metastases for proper management in NT, we investigated new findings for NT using tumor SUVmax value. For a group with high tumor SUVmax, with a value higher than the mean tumor SUVmax, predicting N2 LN metastasis was possible using tumor SUVmax. However, predicting N1 LN was not possible and N1 and N2 LN metastasis for a group with lower tumor SUVmax was not possible. These findings showed that differences in interpretation of N1 and N2 LN status using PET-CT. We attribute these results to the premise that FDG uptake by more advanced lung cancers has better diagnostic accuracy because FDG uptake of the primary lesion positively correlates with LNs (4). And N1 LN is more vulnerable than N2 LN to other conditions such as the shine-through phenomenon, or inflammation.
We found that when SUVmax was equal, pathological stages in NT were significantly higher than non-NT stages and non-NT had significantly higher DFS and OS than NT. These findings might reflect that NT was in more progressive cancer status. Recent studies reported that survival benefits from initial neoadjuvant therapy followed by surgery over initial surgery result from occult metastases undetectable by PET-CT (1,11,17). We attribute our study findings to survival benefits from neoadjuvant therapy followed by surgery.
Restaging LN after neoadjuvant therapy remains controversial (1). PET-CT abnormalities should be confirmed pathologically and more invasive mediastinal LN staging are needed (2,18). However, after neoadjuvant therapy, invasive mediastinal LN staging might be not needed because of frequent discrepancies between PET-CT and pathological findings, especially with previous mediastinal pathological staging (5,10). In addition, some studies show that DFS and OS are sufficiently high to warrant surgery for persistent N2 disease after neoadjuvant therapy (10,11,19-21). We propose with caution that surgery should not be delayed or withheld only based on preoperative PET-CT findings after neoadjuvant therapy, especially after previous LN pathological confirmation. Careful selection of patients who need surgery according to our findings could result in survival benefits after neoadjuvant therapy (17).
In summary, our study suggested similarities and differences in PET-CT findings between NT and non-NT for NSCLC. The similar findings were positive correlation between pathological stage and SUVmax except for N1 LN SUVmax for NT and positive correlation between tumor and LN SUVmax except for tumor and N1 LN for NT. The different findings were the relationship of SUVmax with DFS and OS, prediction of LN metastases, pathological stage when SUVmax values was equal, differences in SUVmax between pathologically malignant and benign LN, and features of N1 and N2 LN. To the best of our knowledge, this study is the first systematic investigation of PET-CT findings between NT and non-NT and differences between N1 and N2 LN in NT. Further prospective large-scale studies on PET-CT findings, especially for NT, are required to derive or establish more definitive findings and guidelines for using PET-CT to evaluate NSCLC.
This study has several limitations, including its retrospective single-center design; lack of ethnic diversity; nonrandomized, heterogeneous data; selection bias, and shine-through phenomenon. Because only surgical cases were included and cases were mostly early-stage, reducing the incidence of LN metastases, preoperative evaluation could have been affected. Because FDG uptake by a lesion varies considerably with conditions, factors can affect SUVmax, especially with early-stage NSCLC. In addition, interpreting SUVmax data with semi-quantitative characteristics using only quantitative standardization could influence preoperative assessment, especially for the early-stage NSCLC. The propensity score matching was used to overcome data heterogeneity.
Conclusions
This study found different findings of PET-CT between NT and non-NT. These differences should be verified for appropriate evaluation and management for NSCLC, especially for surgery planning. SUV max is not a reliable predictor of lymphatic involvement after neoadjuvant therapy in patients with NSCLC. Surgery should not be withheld or delayed based on lack of knowledge about variation in PET-CT findings, which must be carefully interpreted in conjunction with other conditions. In addition, further studies on interpretation of PET-CT finding, especially for NT, are needed for better management and prognosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (IRB Approval number: KC16RIS10839). Written informed consent was given by all patients before PET-CT (Siemens Healthcare, Erlangen, Germany) examinations for potential future use of their clinical data for research.
References
- Lin JT, Yang XN, Zhong WZ, et al. Association of maximum standardized uptake value with occult mediastinal lymph node metastases in cN0 non-small cell lung cancer. Eur J Cardiothorac Surg 2016;50:914-9. [Crossref] [PubMed]
- Yamamoto T, Sakairi Y, Nakajima T, et al. Comparison between endobronchial ultrasound-guided transbronchial needle aspiration and 18F-fluorodeoxyglucose positron emission tomography in the diagnosis of postoperative nodal recurrence in patients with lung cancer. Eur J Cardiothorac Surg 2015;47:234-8. [Crossref] [PubMed]
- Gómez-Caro A, Boada M, Cabañas M, et al. False-negative rate after positron emission tomography/computer tomography scan for mediastinal staging in cI stage non-small-cell lung cancer. Eur J Cardiothorac Surg 2012;42:93-100; discussion 100. [Crossref] [PubMed]
- Eschmann SM, Friedel G, Paulsen F, et al. FDG PET for staging of advanced non-small cell lung cancer prior to neoadjuvant radio-chemotherapy. Eur J Nucl Med Mol Imaging 2002;29:804-8. [Crossref] [PubMed]
- Melloni G, Casiraghi M, Bandiera A, et al. Transbronchial needle aspiration in lung cancer patients suitable for operation with positive mediastinal positron emission tomography. Ann Thorac Surg 2009;87:373-8. [Crossref] [PubMed]
- Al-Sarraf N, Gately K, Lucey J, et al. Clinical implication and prognostic significance of standardised uptake value of primary non-small cell lung cancer on positron emission tomography: analysis of 176 cases. Eur J Cardiothorac Surg 2008;34:892-7. [Crossref] [PubMed]
- Garg PK, Singh SK, Prakash G, et al. Role of positron emission tomography-computed tomography in non-small cell lung cancer. World J Methodol 2016;6:105-11. [Crossref] [PubMed]
- Pan L, Gu P, Huang G, et al. Prognostic significance of SUV on PET/CT in patients with esophageal cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2009;21:1008-15. [Crossref] [PubMed]
- Yang H, Yao F, Zhao Y, et al. Clinical outcomes of surgery after induction treatment in patients with pathologically proven N2-positive stage III non-small cell lung cancer. J Thorac Dis 2015;7:1616-23. [PubMed]
- Kremer R, Peysakhovich Y, Dan LF, et al. FDG PET/CT for assessing the resectability of NSCLC patients with N2 disease after neoadjuvant therapy. Ann Nucl Med 2016;30:114-21. [Crossref] [PubMed]
- Xu YP, Li B, Xu XL, et al. Is There a Survival Benefit in Patients With Stage IIIA (N2) Non-small Cell Lung Cancer Receiving Neoadjuvant Chemotherapy and/or Radiotherapy Prior to Surgical Resection: A Systematic Review and Meta-analysis. Medicine (Baltimore) 2015;94:e879. [Crossref] [PubMed]
- Iskender I, Kadioglu SZ, Cosgun T, et al. False-positivity of mediastinal lymph nodes has negative effect on survival in potentially resectable non-small cell lung cancer. Eur J Cardiothorac Surg 2012;41:874-9. [Crossref] [PubMed]
- Broderick SR, Meyers BF. PET staging of mediastinal lymph nodes in thoracic oncology. Thorac Surg Clin 2012;22:161-6. [Crossref] [PubMed]
- Kubota K, Matsuno S, Morioka N, et al. Impact of FDG-PET findings on decisions regarding patient management strategies: a multicenter trial in patients with lung cancer and other types of cancer. Ann Nucl Med 2015;29:431-41. [Crossref] [PubMed]
- Park JK, Kim JJ, Moon SW. A study about different findings of PET-CT between neoadjuvant and non-neoadjuvant therapy: SUVmax is not a reliable predictor of lymphatic involvement after neoadjuvant therapy for esophageal cancer. J Thorac Dis 2016;8:784-94. [Crossref] [PubMed]
- Carrillo SA, Daniel VC, Hall N, et al. Fusion positron emission/computed tomography underestimates the presence of hilar nodal metastases in patients with resected non-small cell lung cancer. Ann Thorac Surg 2012;93:1621-4. [Crossref] [PubMed]
- Lim HJ, Lee HY, Lee KS, et al. Predictive factors for survival in stage IIIA N2 NSCLC patients treated with neoadjuvant CCRT followed by surgery. Cancer Chemother Pharmacol 2015;75:77-85. [Crossref] [PubMed]
- Sanli M, Isik AF, Zincirkeser S, et al. Reliability of positron emission tomography-computed tomography in identification of mediastinal lymph node status in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;138:1200-5. [Crossref] [PubMed]
- Higgins KA, Chino JP, Ready N, et al. Persistent N2 disease after neoadjuvant chemotherapy for non-small-cell lung cancer. J Thorac Cardiovasc Surg 2011;142:1175-9. [Crossref] [PubMed]
- Macia I, Ramos R, Moya J, et al. Survival of patients with non-small cell lung cancer according to lymph node disease: single pN1 vs multiple pN1 vs single unsuspected pN2. Ann Surg Oncol 2013;20:2413-8. [Crossref] [PubMed]
- Kim JH, Chung WS, Kim YH, et al. Accuracy of Nodal Staging with Integrated PET/CT Scanning in Non-small Cell Lung Cancer. Korean Journal of Thoracic and Cardiovascular Surgery 2010;43.


