Postoperative change of the psoas muscle area as a predictor of survival in surgically treated esophageal cancer patients
Introduction
Esophageal cancer is one of the most aggressive and deadly cancers and has a dismal prognosis. Current operative strategies based on esophagectomy have increased the survival rate despite its invasiveness (1). However, because esophageal resection for esophageal cancer involves extensive resection and reconstruction of the upper gastrointestinal tract, there is a resultant risk of postoperative malnutrition with significant digestive problems such as reduced gastric volume, delayed food intake, impaired gastric emptying, dysphagia, and gastro-esophageal reflux (2,3). Postoperative weight loss is also a frequent outcome; a previous study reported that a fifth of patients lose at least 20 per cent of their preoperative weight within 6 months after esophagectomy (3).
Malnutrition has been thought to be related to poor immediate postoperative outcomes and survival after esophagectomy (1,4). Several markers have been applied to detect malnutrition, such as serum albumin, thickness of the triceps, and weight loss. Sarcopenia has recently been suggested as a concept that can also reflect malnutrition. Sarcopenia is a syndrome characterized by the progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes such as physical disability, poor quality of life, and death (5). Among its various characteristics, the loss of skeletal muscle mass was identified as a poor prognostic factor for patients with various cancers, such as pancreatic cancer, colorectal cancer with liver metastasis, and melanoma (6-8). Several studies also showed that the preoperative psoas muscle area (PMA), which, because it is a core muscle, can reflect the status of skeletal muscle in the whole body, is related to survival after esophagectomy (9,10). We considered that the PMA could change after surgery due to postoperative malnutrition and weight loss. We therefore hypothesized that a further decrement in the PMA after surgery, in addition to a preoperative decreased PMA, might be related to poor survival after esophagectomy in esophageal cancer patients. This study was performed to investigate the change of the PMA one year after esophagectomy and its prognostic role in patients with surgically treated esophageal cancer.
Methods
Patients
The Institutional Review Board of our hospital approved this retrospective study, and the requirement to obtain informed consent was waived (AJIRB-MED-MDB-16-049). A total of 58 patients who underwent transthoracic esophagectomy with extensive lymphadenectomy and esophagogastrostomy for esophageal cancer at Ajou University Hospital between April 2004 and December 2013 were analyzed. Those patients who experienced operative mortality, death, or recurrence within one year were excluded from the analysis. We retrospectively reviewed the medical records and pathological data of the study patients. Chest and abdominal computed tomography (CT) scans and 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography (PET)/CT scans were taken for preoperative diagnosis. All patients were staged using the American Joint Committee on Cancer criteria (7th edition) (11). Chest and abdominal CT scans were obtained at 6-month intervals and PET/CT scans were obtained annually to detect recurrences after surgery. Locoregional recurrence was defined as recurrence at an anastomosis site, the mediastinum, or the abdomen where lymph nodes were dissected. Distant recurrences were defined as those occurring outside the operative field, such as in the lung, brain, liver, adrenal glands, bone, or another location. Recurrence was diagnosed based on the imaging results, and tissue biopsies were taken of suspected recurrent lesions, where possible.
Measurement of the PMA
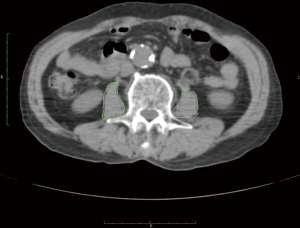
The PMA was measured on an axial CT section that was obtained during abdominal CT or PET/CT imaging (Figure 1). Polygonal regions of interest were drawn on both psoas muscles, where both pedicles of the third lumbar vertebrae were visible, using the Osirix software (ver. 7.0; Pixmeo, Bernex, Switzerland) with a predetermined density threshold (40–400 Hounsfield units). The area (cm2) was calculated automatically by the software. The preoperative PMA was measured on preoperative CT images and the postoperative PMA was measured on the one-year follow-up CT images. The percentage change of the PMA was calculated as follows: delta (%) = (postoperative PMA − preoperative PMA) / preoperative PMA × 100.
Statistical analysis
Statistical analysis was performed using the open source statistical software R (http://www.R-project.org) and the STATA 11 software (Stata Statistical Software, Release 11 [2005]; Stata Corp., College Station, TX, USA). Clinical and pathological parameters are described as means ± standard deviations for continuous variables and as frequencies (%) for categorical variables. Overall survival (OS) was measured from the date of the operation to the date of death from any cause or the last clinical follow-up. Disease-free survival (DFS) was measured from the date of the operation to the date of recurrence or the last clinical follow-up. The Kaplan–Meier method and log-rank test were used to perform the univariate survival analysis, and Cox’s proportional hazard model was used to identify independent prognostic factors. Survival curves were estimated using the Kaplan–Meier method, and differences in survival between the groups were analyzed using the log-rank test. All of the tests were two-sided and a P value <0.05 was considered to be statistically significant.
Results
General patient characteristics and patterns of recurrence
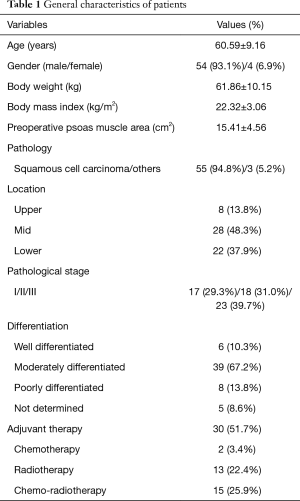
The 58 study patients included 54 male (93.1%) and 4 female (6.9%) patients with a mean age of 60.59±9.16 years (Table 1), and the median follow-up period was 20.52 months. The mean preoperative body weight and body mass index (BMI) were 61.86±10.15 kg and 22.32±3.06 kg/m2, respectively. Lesions were located mainly in the mid- (28, 48.3%) or lower (22, 37.9%) esophagus. A total of 17 patients (29.3%) had Stage I disease, 18 (31.0%) had Stage II, and 23 (39.7%) had Stage III.
Full table
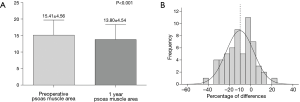
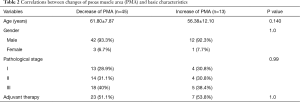
The mean preoperative PMA was 15.41±4.56 cm2 and the mean postoperative one-year PMA was 13.80±4.54 cm2. The PMA was significantly decreased after surgery (Figure 2A, P<0.001). The mean delta was −10.17%. Forty-five (77.6%) patients showed a decreased PMA, whereas 13 (22.4%) patients showed an increased PMA (Figure 2B). In Table 2, the correlations between changes of PMA and patients’ basic characteristics were compared and changes of PMA were not related to the patients’ basic characteristics. Patients’ body weight was found to be significantly decreased after surgery (61.86±10.15 vs. 55.84±9.06 kg, P<0.001) and the mean change in body weight was −9.39%. Fifty-one (89.7%) patients showed a decreased body weight, whereas only 6 (10.3%) patients showed an increased body weight.
Full table
Half (29, 50%) of all patients suffered from recurrence. Locoregional recurrence developed in 22 (37.9%) patients. Distant metastases were detected in 7 (12.1%) patients: 5 in the lungs, 1 in the liver, and 1 in bone.
Survival analysis and risk factors for OS
Univariate analysis revealed that the pathological stage, preoperative PMA, and delta were risk factors for OS (Table 3). On multivariate analysis, the preoperative PMA [hazard ratio (HR) =0.819, P=0.002], delta (HR =0.688, P=0.001), and pathologic stage (Stage III vs. I, HR =3.388, P=0.016) were also risk factors for OS.
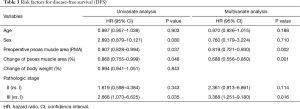
Full table
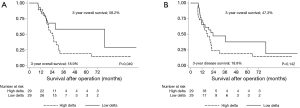
Using the mean delta of −10% as a cut-off, the patients were divided into two groups: the high delta group (PMA decreased by more than 10%, n=29) and low delta group (PMA decreased by less than 10% or increased, n=29). The 3-year OS for the high and low delta groups were 18.9% and 58.2% (P=0.049, Figure 3A), respectively. The 3-year DFS for the high and low delta groups were 18.8% and 47.3% (P=0.142, Figure 3B), respectively.
Discussion
This study showed that the psoas muscle mass was significantly decreased after esophagectomy. Both the decreased preoperative PMA and the decrease in the PMA during the one-year follow-up after surgery were related to poor survival after surgical treatment of esophageal cancer.
Upper gastrointestinal surgeries such as esophagectomy and gastrectomy have been shown to cause postoperative malnutrition (12). In particular, esophageal resection for esophageal cancer involves extensive resection and reconstruction of the upper gastrointestinal tract and can result in significant digestive problems such as reduced gastric volume, delayed food intake, impaired gastric emptying, dysphagia, gastro-esophageal reflux, and postoperative malnutrition (2,3). Although many parameters such as a change in body weight, triceps skin folds, mid-arm muscle mass, and grip strength could be applied to malnutrition, weight loss is a prominent indicator that reflects malnutrition after upper gastrointestinal surgery (12). A previous study reported that a fifth of patients lose at least 20 per cent of their preoperative weight within 6 months after esophagectomy (3). Postoperative weight loss is also related to reduced quality of life, and is mainly associated with meal size and gastrointestinal symptoms (12). In addition, D’Journo et al. reported that postoperative weight loss is also related to DFS; weight loss exceeding 10% of the initial body weight over the course of one year has a negative prognostic impact on DFS after esophagectomy for esophageal cancer. Postoperative malnutrition is an important clinical problem after esophagectomy and is associated with both quality of life and survival after this procedure. In addition, the reduced nutritional status affects the patient’s tolerance for further treatments, should they be required (13-15).
Despite the fact that weight loss is frequently encountered after upper gastrointestinal surgery such as esophagectomy, it is not clear which body composition components are changed after surgery. In gastrectomy patients, body composition changes have been reported that have created considerable controversy. Some researchers found large losses in fat mass but only small losses in muscle (16), while others observed significant losses in both muscle and fat (17). However, such changes in body composition have not been studied in esophagectomy patients. From our data, the mean delta was −10.17% and 45 (77.6%) patients showed a decreased PMA. This indicates that muscle mass was also significantly decreased after esophagectomy.
Sarcopenia is a recently suggested concept that can reflect malnutrition. The definition of sarcopenia is based on documented low muscle mass with low muscle strength or reduced physical performance (5). Although sarcopenia has been regarded as being related to the natural process of aging, it is also relevant in the diminished homeostatic reserve observed in malnutrition, chronic illnesses, and organ failure (18). Several retrospective studies have focused only on the loss of muscle area. In particular, the psoas muscle has been used as an indicator of muscle mass because it is a core muscle that is relatively uninfluenced by deliberate weight training and exercise and is not surrounded by other muscles or bony anatomy that would obscure the isolation of the muscle from structures of similar radiodensity (9). Unlike previous studies on gastrectomy and malnutrition that evaluated the change in muscle mass by measuring the thickness of the triceps, we instead measured the PMA. We considered that the psoas muscle could reflect sarcopenia better than triceps thickness because the thickness of the triceps could be altered by exercise or training. Results from our data revealed that both the preoperative PMA and the change in PMA during the 1-year follow-up were related to survival. With regard to weight loss, our results are discordant with previous studies that demonstrated that weight loss after surgery was related to OS (1). This discrepancy can be explained by our small study population. However, our results indicate that muscle area plays a more important role than body weight. For example, in the case of sarcopenic obesity, body weight and BMI could be normal or increased even though the muscle volume is decreased.
This study has clinical implications. Because postoperative malnutrition is related to poor survival, surgeons and clinicians should pay attention to the postoperative nutritional status of their patients. A special program on nutritional support in post-esophagectomy patients is mandatory (1). Abdominal CT or PET/CT is frequently performed during the follow-up of esophagectomy patients for the surveillance of recurrence. Clinicians can also use abdominal CT or PET/CT to measure changes in the PMA as a marker of postoperative nutritional status. Several studies that have been performed to evaluate the effect of nutritional intervention on caloric and proteinic ingestion in several cancer models have found that specialized nutritional support can enhance many of the parameters that predict a poor outcome (19,20). In addition, it is well known that perioperative immunonutrition with enriched enteral nutrition modulates the immune function, limits catabolism in patients with advanced cancer with preservation of lean body mass and muscle mass, and is likely to improve survival (21,22). These specialized nutritional supports have to be studied during the postoperative period after esophagectomy and the PMA could be applied as a marker of malnutrition. Our next goal is to study the effects of enteral nutrition such as jejunostomy feeding during the immediate postoperative period and its effect on long-term survival.
This study has some limitations. First, we measured the PMA only one year after the operation. A previous study reported that postoperative weight loss appeared to be stable between 6 months and 1 year (1); likewise, the PMA might also be stable within 6–12 months after surgery. Some patients did not take the abdomen CT or PET CT before the one year follow0up because the follow-up strategies after esophagectomy was not established in early series, therefore the time trends of PMA could not be analyzed in this data. Second, the results of this study are based on a small sample size. A multicenter study with larger numbers of patients is, thus, required. Third, we did not measure the other parameters on nutrition such as triceps skin fold and mid-arm muscle. In further study, we will collect the data on other parameters of nutrition, and we will compare these in further study. In addition, the data of ‘quality of life’ after operation also could be analyzed with data of PMA in the future.
In conclusion, muscle mass revealed by the PMA was significantly decreased after esophagectomy. The decrease in the PMA had a negative prognostic effect on OS in patients with surgically treated esophageal cancer. Preservation of the PMA during the postoperative period might improve the long-term outcomes.
Acknowledgements
This work was supported by the faculty research fund of Ajou University School of Medicine.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board of our hospital approved this retrospective study, and the requirement to obtain informed consent was waived (AJIRB-MED-MDB-16-049).
References
- D'Journo XB, Ouattara M, Loundou A, et al. Prognostic impact of weight loss in 1-year survivors after transthoracic esophagectomy for cancer. Dis Esophagus 2012;25:527-34. [Crossref] [PubMed]
- Martin L, Jia C, Rouvelas I, et al. Risk factors for malnutrition after oesophageal and cardia cancer surgery. Br J Surg 2008;95:1362-8. [Crossref] [PubMed]
- Martin L, Lagergren J, Lindblad M, et al. Malnutrition after oesophageal cancer surgery in Sweden. Br J Surg 2007;94:1496-500. [Crossref] [PubMed]
- Saito T, Zeze K, Kuwahara A, et al. Correlations between preoperative malnutrition and septic complications of esophageal cancer surgery. Nutrition 1990;6:303-8. [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- van Vledder MG, Levolger S, Ayez N, et al. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 2012;99:550-7. [Crossref] [PubMed]
- Sabel MS, Lee J, Cai S, et al. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol 2011;18:3579-85. [Crossref] [PubMed]
- Tan BH, Birdsell LA, Martin L, et al. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 2009;15:6973-9. [Crossref] [PubMed]
- Sheetz KH, Zhao L, Holcombe SA, et al. Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus 2013;26:716-22. [PubMed]
- Park SY, Yoon JK, Lee SJ, et al. Prognostic value of preoperative total psoas muscle area on long-term outcome in surgically treated oesophageal cancer patients. Interact Cardiovasc Thorac Surg 2017;24:13-19. [Crossref] [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Carey S, Storey D, Biankin AV, et al. Long term nutritional status and quality of life following major upper gastrointestinal surgery - a cross-sectional study. Clin Nutr 2011;30:774-9. [Crossref] [PubMed]
- Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491-7. [Crossref] [PubMed]
- Andreyev HJ, Norman AR, Oates J, et al. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 1998;34:503-9. [Crossref] [PubMed]
- Polee MB, Hop WC, Kok TC, et al. Prognostic factors for survival in patients with advanced oesophageal cancer treated with cisplatin-based combination chemotherapy. Br J Cancer 2003;89:2045-50. [Crossref] [PubMed]
- Liedman B, Andersson H, Bosaeus I, et al. Changes in body composition after gastrectomy: results of a controlled, prospective clinical trial. World J Surg 1997;21:416-20; discussion 420-1. [Crossref] [PubMed]
- Bae JM, Park JW, Yang HK, et al. Nutritional status of gastric cancer patients after total gastrectomy. World J Surg 1998;22:254-60; discussion 260-1. [Crossref] [PubMed]
- Cesari M, Leeuwenburgh C, Lauretani F, et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr 2006;83:1142-8. [PubMed]
- Read JA, Beale PJ, Volker DH, et al. Nutrition intervention using an eicosapentaenoic acid (EPA)-containing supplement in patients with advanced colorectal cancer. Effects on nutritional and inflammatory status: a phase II trial. Support Care Cancer 2007;15:301-7. [Crossref] [PubMed]
- Gonçalves Dias MC, de Fátima Nunes Marucci M, Nadalin W, et al. Nutritional intervention improves the caloric and proteic ingestion of head and neck cancer patients under radiotherapy. Nutr Hosp 2005;20:320-5. [PubMed]
- Ryan AM, Reynolds JV, Healy L, et al. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg 2009;249:355-63. [Crossref] [PubMed]
- Gianotti L, Braga M, Nespoli L, et al. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology 2002;122:1763-70. [Crossref] [PubMed]