Effects of leukotriene D4 nasal challenge on bronchial responsiveness and inflammation in asthmatic patients with allergic rhinitis
Introduction
The prevalence of coexisting allergic rhinitis (AR) and asthma is increasing worldwide (1,2). AR, particular severe and persistent AR, facilitates the development and worsens of asthma control (3). Bronchial hyperresponsiveness (BHR) may have existed in patients with AR who had no clinical manifestation of asthma. BHR reportedly increased after nasal allergen challenges in patients with AR with or without coexisting asthma (4-7).
BHR has been associated with airway eosinophilia (8). Cysteinyl leukotrienes (CysLTs) are potent lipid mediators which recruit eosinophils and eosinophil progenitors from bone marrow to airways in allergic diseases (9,10). The increased production of CysLTs in the secretion of upper and lower airways has been shown to elicit BHR after nasal allergen challenges (8,9,11). However, the role of CysLTs in increasing BHR after nasal allergen challenge has not been evaluated.
The aim of this study was to evaluate the effects of LTD4 nasal challenge on BHR and airway inflammation in asthmatic patients with AR.
Methods
The study protocol (NCT01963741) was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University. Written informed consent was signed prior to the study.
Participants
Asthmatic patients with AR of either gender, aged 18 to 50 years were consecutively recruited from the First Affiliated Hospital of Guangzhou Medical University between March 2013 and April 2014. The participants tested positive to methacholine (Mch) bronchial provocation test, and had recurrent nasal symptoms (sneezing, nasal discharge, nasal blockage or itching) and lower airway symptoms (cough, breathlessness, chest tightness, wheezing, etc.) in the preceding year. All subjects also tested positive to at least one of the panel of aeroallergens by using skin prick testing. The diagnosis of AR and asthma were made according to the international guidelines (ARIA and GINA) (12,13). Patients who underwent immunotherapy, had acute upper or lower airway infection within the previous 4 weeks, had any other respiratory disease (e.g., bronchiectasis, chronic obstructive pulmonary disease), were during pregnancy or lactation, and currently smoking were excluded. Antihistamines, leukotrienes receptor antagonists and inhalation or systemic corticosteroids were withheld for at least 2 weeks prior to the study.
Study design
After screening, participants attended the research center on 3 consecutive days. During the first visit, anterior rhinoscopy, fractional exhaled nitric oxide (FeNO), nasal lavage, spirometry and Mch bronchial provocation test (Jaeger, Germany), and induced sputum (30 minutes after bronchial provocation test, when forced expiratory flow in one second (FEV1) restored to baseline after inhalation of 200 µg salbutamol) were performed. In the second visit, participants underwent FeNO measurement, LTD4 nasal challenge, Mch bronchial provocation test (30 minutes after LTD4 nasal challenge), nasal lavage, and induced sputum (30 minutes after the bronchial provocation test when FEV1 recovered to pre-challenge level following inhalation of 200 µg salbutamol). In the third visit (24 hours after nasal challenge test), all subjects underwent FeNO, nasal lavage, spirometry, and induced sputum tests.
Measurements
Measurements were taken indoors with the room temperature between 20 and 25 °C and a constant humidity in the morning (8:00–12:00).
Prior to nasal challenge, there was a 30-minute acclimatization period for each participant. LTD4 nasal challenge tests were performed as described previously (14). Briefly, the diluents {[4–16]×10−3mg·mL−1} were delivered via nasal spray in a step-wise manner with the rate of increase in nasal airway resistance (NAR) and induced symptom scores as the measurement outcomes (15). NAR was measured with an active anterior rhinomanometry by using rhinomanometer (Jaeger, Germany) according to international guideline (16). The procedures were terminated in case of a 60% increase in NAR and/or a composite symptom score reached to greater than 3 points (0 point for sneezing <3; 1 point for the score of 3–5; 2 points for the score of >5; 0 point for no rhinorrhea; 1 point for mild (<1 mL) rhinorrhea; 2 points for abundant (>1 mL) rhinorrhea; 0 point for no pruritus; 1 point for mild pruritus (palate, eyes or throat); 2 points for severe pruritus (conjunctivitis, cough, urticaria or difficult breathing) was reached or until the use of the final concentration of LTD4 diluent.
FeNO was measured by using NIOX MINO (Aerocrine, Sweden) according to the American Thoracic Society (ATS) guideline (17); lung function and Mch bronchial provocation tests were performed (Jaeger, Germany) according to ATS/European Respiratory Society (ERS) guidelines (18,19). All instruments met the standard guidelines of ATS/ERS and were calibrated each day.
Nasal lavage was performed with the patient’s head forward. A 10 mL saline solution was injected into the nostrils and the lavage fluid was recovered. The nasal lavage fluid was immediately centrifuged, and cells were re-suspended for cytology staining. Differential cells counts were performed on haematoxylin-eosin stained slides. Sputum was induced by 3–5% hypertonic saline which was nebulized for 20 minutes. Cell viability was determined using the trypan blue staining approach. Samples with cell viability >70% and squamous cell <20% were considered of adequate quality. Differential inflammatory cells counts were performed by counting 400 cells on haematoxylin-eosin slides.
Statistical analysis
Statistical analysis was performed by using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Data with normal distribution were expressed as mean ± standard deviation 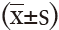 ; otherwise median (interquartile range). Paired t-tests and one-way ANOVA tests were performed for comparison of the differences of PD20FEV1-Mch, FeNO, and inflammatory cells counts in nasal lavage and sputum before and after nasal challenges.
; otherwise median (interquartile range). Paired t-tests and one-way ANOVA tests were performed for comparison of the differences of PD20FEV1-Mch, FeNO, and inflammatory cells counts in nasal lavage and sputum before and after nasal challenges.
Results
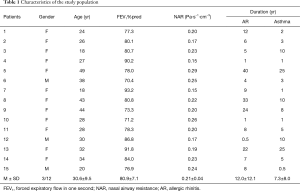
A total of 18 subjects were enrolled in this study, 3 subjects withdrew due to their unwillingness to complete the LTD4 nasal challenge or Mch bronchial provocation tests. Fifteen asthmatic patients with AR (male/female: 3/12 cases) completed the study (Table 1).
Full table
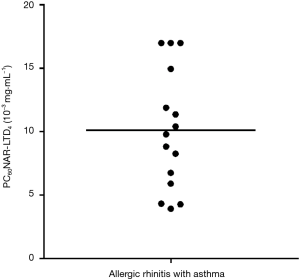
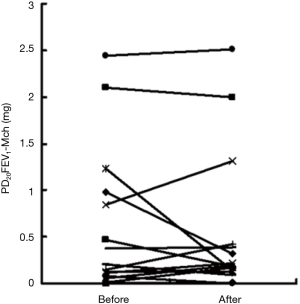
Dust mites were the major category of allergens tested in this study (10/15 of subjects were sensitive to house dust mite). In total, 80% (12/15) of subjects tested positive to LTD4 nasal challenge with a mean PC60NAR of (8.39±3.48)×10−3 mg·mL−1 (Figure 1) and induced symptom score of 0.75±1.29. The distribution of PD20FEV1-Mch before and after LTD4 nasal challenges are shown in Figure 2. PD20FEV1-Mch decreased non-significantly after LTD4 nasal challenges (3.05±3.81 vs. 2.70±3.81 µmol, P=0.45). There were no significant changes in FEV1, maximum mid-expiratory flow (MMEF), maximal expiratory flow at 50% (MEF50%), and maximal expiratory flow at 25% (MEF25%) before and after LTD4 nasal challenges (Table 2).
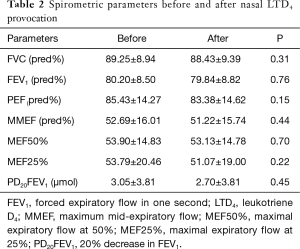
Full table
The eosinophil counts were (38.36±23.14)% and (45.70±24.86)% in nasal lavage, and (17.51±11.05)% and (24.29±16.52)% in induced sputum before and 24 hours after nasal challenge. No significant correlation was found between the changes in sputum eosinophil counts and the changes in PD20FEV1 for Mch after nasal challenge (Pearson correlation: r=−0.418, P=0.155). There were no significant changes of inflammatory cell counts in nasal lavage and induced sputum before and after LTD4 nasal challenge (P>0.05). The values of FeNO, eosinophil counts and neutrophil counts in nasal lavage and induced sputum before and after LTD4 nasal challenges are shown in Table 3.

Full table
Discussion
In this study, most subjects (80%) tested positive to LTD4 nasal challenge with the nasal responsiveness (increased nasal resistance and more prominent symptoms) consistent with those reported in previous studies (20,21), which supported the theory that CysLTs play a vital role in the pathogenesis of AR. Moreover, it has also been proven that LTD4 effectively elicited bronchospasm in asthmatic patients in an attempt to identify leukotriene responsiveness subtypes (22,23). AR and asthma is a continuum of the inflammation involving one common airway (2), and the patients with coexisting AR and asthma might be the ideal candidates for initiating anti-leukotriene therapy (24,25).
LTD4 nasal provocation tests were safe for asthmatics, because LTD4 would mostly be deposited in the upper airways when properly performed. In our study, no significant differences were found in spirometric parameters (FEV1, MMEF, MEF50% and MEF25%) before and after LTD4 nasal challenges, which was consistent with the previous findings (26). Our results showed that the PD20FEV1-Mch decreased non-significantly after LTD4 nasal challenges. Corren and his colleagues have reported that nasal challenge with allergen might induce an increase in BHR to Mch compared to that of placebo (6). Our data suggested that although CysLTs play a vital role in the pathogenesis of AR and asthma, it may not be capable of increasing the BHR after nasal allergen challenge. Neurogenic inflammation, nasal-bronchial reflex and other factors may have accounted for the increase in BHR after nasal allergen challenges (27).
CysLTs are potent lipid mediators which recruit inflammatory cells in AR and asthma (9,28). In the present study, the percentages of eosinophils in sputum and nasal lavage increased non-significantly after nasal LTD4 challenge. It also has been reported that, compared with allergens, LTD4 inhalation challenge did not increase the number of sputum eosinophils in asthmatic patients (28). Several reasons may have accounted for the BHR after nasal allergen challenge. First, the season of nasal challenge or natural allergen exposure may have activated the airway inflammatory cells, as reported by Marcucci et al. (26). Second, the significant local nasal inflammation leading to a generalized systemic immune stimulation is essential for the increase in bronchial responsiveness. Third, the provocative agents or allergens may have stimulated the inflammatory cells after nasal challenge (29). Apart from these, the percentages of inflammatory cells may vary with time after nasal challenges, however, in this study the inflammatory cells have not been measured continually at different time points after nasal challenge.
There were several limitations of this study. First, the sample size might not be sufficiently powered for comparisons of all individual parameters. Second, the inflammatory mediators in nasal secretions were not assessed at different time points after nasal challenges. Third, we lacked a control group of patients with AR without asthma which could be useful to investigate how LTD4 nasal challenge impacts on airway inflammatory cell counts.
In conclusion, although the majority of asthmatic patients with AR tested positive to LTD4 nasal challenge, no remarkable difference in BHR could be observed after nasal challenge. LTD4 might not have elicited an increase in bronchial responsiveness following nasal allergen challenge. Eosinophil recruitment after LTD4 nasal challenge needs to be further studied in asthma.
Acknowledgements
We thank Dr. Shi Xu, Qingxia Liu, E Guo, Linting Luo, Diteng Luo, Xiangdong Xu, Huayi Huang, Yongqing Ye and Xianmiao Ye for their assistance of recruiting participants.
Funding: This study was supported by The National Key Scientific & Technology Support Program (No. 2015BAI12B10), National Natural Science Youth Foundation of China (No. 81300017).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. NCT01963741) and written informed consent was obtained from all patients.
References
- Zhang Y, Zhang L. Prevalence of Allergic Rhinitis in China. Allergy Asthma Immunol Res 2014;6:105-13. [Crossref] [PubMed]
- Grossman J. One Airway, One Disease. Chest 1997;111:11S-16S. [Crossref] [PubMed]
- Lin J, Su N, Liu GL, et al. The impact of concomitant allergic rhinitis on asthma control: a cross-sectional nationwide survey in China. J Asthma 2014;51:34-43. [Crossref] [PubMed]
- Bonay M, Neukirch C, Grandsaigne M, et al. Changes in airway inflammation following nasal allergic challenge in patients with seasonal rhinitis. Allergy 2006;61:111-8. [Crossref] [PubMed]
- Linneberg A, Henrik NN, Frølund L, et al. The link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen Allergy Study. Allergy 2002;57:1048-52. [Crossref] [PubMed]
- Corren J, Adinoff AD, Irvin CG. Changes in bronchial responsiveness following nasal provocation with allergen. J Allergy Clin Immunol 1992;89:611-8. [Crossref] [PubMed]
- Wang DY, Goh DY, Ho AK, et al. The upper and lower airway responses to nasal challenge with house-dust mite Blomia tropicalis. Allergy 2003;58:78-82. [Crossref] [PubMed]
- de Graaf-in t Veld C, Garrelds IM, Koenders S, et al. Relationship between nasal hyperreactivity, mediators and eosinophils in patients with perennial allergic rhinitis and controls. Clin Exp Allergy 1996;26:903-8.
- Saito H, Morikawa H, Howie K, et al. Effects of a cysteinyl leukotriene receptor antagonist on eosinophil recruitment in experimental allergic rhinitis. Immunology 2004;113:246-52. [Crossref] [PubMed]
- Boulay ME, Duchesneau E, Jacques E, et al. CysLT1-R expression following allergen provocation in asthma and allergic rhinitis. Prostaglandins Leukot Essent Fatty Acids 2010;83:15-22. [Crossref] [PubMed]
- Hens G, Raap U, Vanoirbeek J, et al. Selective Nasal Allergen Provocation Induces Substance P-Mediated Bronchial Hyperresponsiveness. Am J Respir Cell Mol Biol 2011;44:517-23. [Crossref] [PubMed]
- Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 Update. Allergy 2008;63:8-160. [Crossref] [PubMed]
- From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2012. Available online: http://www.ginasthma.org/
- Zhu Z, Xie Y, Guan W, et al. Leukotriene D4 nasal provocation test: Rationale, methodology and diagnostic value. Exp Ther Med 2016;12:525-9. [PubMed]
- Riechelmann H, Bachert C, Goldschmidt O, et al. Application of the Nasal Provocation Test on Diseases of the Upper Airways Position Paper of the German Society for Allergology and Clinical Immunology (ENT Section) in Cooperation with the Working Team for Clinical Immunology. Laryngorhinootologie 2003;82:183-8. [Crossref] [PubMed]
- Clement PA, Gordts F. Standardisation Committee on Objective Assessment of the Nasal Airway, IRS, and ERS. Consensus report on acoustic rhinometry and rhinomanometry. Rhinology 2005;43:169-79. [PubMed]
- Spector SL, Nicklas RA, Chapman JA, et al. Symptom severity assessment of allergic rhinitis: part 1. Ann Allergy Asthma Immunol 2003;91:105-14. [Crossref] [PubMed]
- American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912-30. [Crossref] [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS task force: Standardization of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Bisgaard H, Olsson P, Bende M. Effect of leukotriene D4 on nasal mucosal blood flow, nasal airway resistance and nasal secretion in humans. Clin Allergy 1986;16:289-97. [Crossref] [PubMed]
- Okuda M, Watase T, Mezawa A, et al. The role of leukotriene D4 in allergic rhinitis. Ann Allergy 1988;60:537-40. [PubMed]
- Guan W, Zheng JP, Gao Y, et al. Leukotriene D4 bronchial provocation test: methodology and diagnostic value. Curr Med Res Opin 2012;28:797-803. [Crossref] [PubMed]
- Guan WJ, Zheng JP, Gao Y, et al. Leukotriene D4 and methacholine bronchial provocation test for identifying leukotriene responsiveness subtypes. J Allergy Clin Immunol 2013;131:332-8.e1. [Crossref] [PubMed]
- Price DB, Swern A, Tozzi CA, et al. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy 2006;61:737-42. [Crossref] [PubMed]
- Virchow JC, Bachert C. Efficacy and safety of montelukast in adults with asthma and allergic rhinitis. Respir Med 2006;100:1952-9. [Crossref] [PubMed]
- Marcucci F, Passalacqua G, Canonica GW, et al. Lower airway inflammation before and after house dust mite nasal challenge: An age and allergen exposure-related phenomenon. Respir Med 2007;101:1600-8. [Crossref] [PubMed]
- Corren J. Allergic rhinitis and asthma: how important is the link? J Allergy Clin Immunol 1997;99:S781-6. [Crossref] [PubMed]
- Mulder A, Gauvreau GM, Watson RM, et al. Effect of Inhaled Leukotriene D4 on Airway Eosinophilia and Airway Hyperresponsiveness in Asthmatic Subjects. Am J Respir Crit Care Med 1999;159:1562-7. [Crossref] [PubMed]
- Bergmann-Hug K, Wirth R, Henseler M, et al. Effect of natural seasonal pollen exposure and repeated nasal allergen provocations on elevation of exhaled nitric oxide. Allergy 2009;64:1629-34. [Crossref] [PubMed]


