Erroneous diagnosis of small cell lung cancer based on small biopsies with far-reaching consequences: case report of a typical carcinoid tumor
Introduction
Small cell lung carcinoma (SCLC) is the most common neuroendocrine (NE) lung carcinoma, representing approximately 14% of invasive lung cancers, whereas carcinoid tumors account for 1% to 2%. Patients with typical carcinoid (TC) tumors are younger than those with SCLC, with a median age of 48 vs. 70, respectively (1). SCLC is related to heavy smoking, whereas TC is not (2). Pathologically, NETs are primarily defined according to the morphology of neoplasm characterized by monotonous, monomorphic neoplastic cells organized in repetitive organoid or trabecular pattern in absence of intratumoral necrosis and/or nuclear atypia based on light microscopy. Mitotic index is considered prevalently for classificative purposes (3,4). Immunohistochemical stains are needed for SCLC diagnosis and the panel should include chromogranin A, Ki67 and synaptophysin (5,6). The basic treatment is surgery for TC and chemotherapy for SCLC.
Case presentation
A 46-year-old non-smoking female patient was diagnosed with central right-sided SCLC in July 2014 (Figure 1A). The initial stage was cT3, cN1, cM0, and the patient complained of fatigue and dyspnea. The diagnosis was established by endobronchial biopsy. The tumor cells were positive for chromogranin A, synaptophysin, and CD56, and negative for cytokeratin 5/6, LCA, p63, and TTF-1. Ki67 staining was not performed. Treatment was initiated with six cycles of cisplatin and etoposide and 60 Gy of thoracic irradiation simultaneously, followed by prophylactic cranial irradiation (PCI; 30.6 Gy). Re-staging during treatment and after treatment completion showed no change at the primary site. One year later, PET-CT demonstrated local tumor progression (Figure 1B). The patient then came for a second opinion at our tertiary referral clinic.
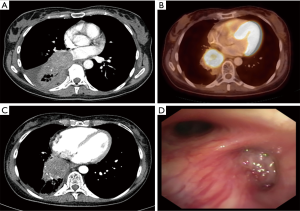
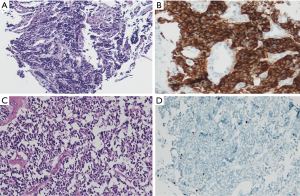
At the date of admission, the patient was in good general condition with some peripheral neuropathy from chemotherapy. She reported a decline in psychomotor agitation in the last six months after PCI with persistently impaired thinking. Otherwise, her quality of life was good. The reevaluation with a CT scan and endobronchial ultrasound transbronchial aspiration (EBUS-TBNA) demonstrated non-suspicious mediastinal nodes and complete occlusion of the bronchus of the right lower lobe (Figure 1C,D). New bronchoscopic biopsies showed a weakly proliferating NET, without squeezing artifacts as in the primary biopsies (Figure 2A). After case discussion in the multidisciplinary tumor conference, we performed an extended right sided pneumonectomy with partial resection of the left atrium and pericardium. The bronchial stump was covered with the azygos vein. The postoperative course was uneventful. The patient was discharged on postoperative day 12 in good condition. A histopathological examination confirmed the diagnosis of a regressively altered TC without necrosis with a maximum size of 5.5 cm, with severe scarring in the border zone and marked peribronchial fibrosis. Immunohistochemistry was strongly positive for chromogranin A (Figure 2B,C), positive for synaptophysin and negative for TTF-1. Ki67 staining performed in the preoperative biopsies (Figure 2D) showed <10 positive cells and was not repeated in the resected specimen. One mitosis per 10 high power field was found. The pathological stage was ypT2b, ypN0 (0/7), M0, L0, V0, R0, G1. The nodal dissection included paraesophageal, subcarinal, hilar and peribronchial (three) lymph nodes. No residual tumor was found in the left atrium or pericardium; the partial resection was necessary due to extensive postradiogenic adhesions. A follow-up examination six weeks later showed no complications. No signs of tumor recurrence were found by CT eight months postoperatively (Table 1).
Full table
Discussion
This young patient had a misdiagnosis of SCLC followed by aggressive treatment including PCI. This treatment did not induce TC remission and led to well-known side effects such as neuropathy and possible psycho-intellectual impairment. While only a subset of pulmonary carcinoid tumors, especially atypical carcinoids (ACs), are responsive to chemotherapy, TC normally does not react (7). Retrospectively, an initial lower bilobectomy would have been possible in this case. Unfortunately, avoiding pneumonectomy after definitive chemo-radiotherapy was not possible.
The misleading pathological diagnosis resulted from crush artifacts in tumor cells, which commonly occur in very small biopsies (8). It also resulted from incomplete immunohistochemistry since Ki67 staining was not performed, and due to the fact that the clinical circumstances were not questioned. Despite crush artifacts, only one biopsy could be enough to obtain the right diagnosis between TC and SCLC after studying the Ki67 distribution in the neoplastic cells. Most SCLC show extensive necrosis and a high mitotic rate of 60 to 80 mitoses per high power field (HPF), which is used to distinguish SCLC from other NETs. TC demonstrates fewer than two mitoses per HPF and no necrosis. Furthermore, TC can be separated from SCLC by Ki67 staining, which is of special importance because it indicates the low proliferation rate of less than 5% in TC (Figure 2D), whereas it is usually as high as 70–100% in SCLC (5,6). Generally, immunohistochemistry can be very helpful to define NETs with chromogranin A and synaptophysin, in this setting.
Despite the fact that the patient was young and a non-smoker, the clinicians did not doubt the histopathologic diagnosis. Therefore, we underline the importance of the clinical aspects before establishing a diagnosis of SCLC. In this case, the tumor progressed to a small degree during chemo-radiotherapy. Importantly, when an assumed SCLC does not react to first-line chemotherapy, one should reevaluate the situation by obtaining new tumor biopsies. There are very few data to support the use of definitive chemoradiation for TC. Definitive radiation should be considered only in locally advanced unresectable TC (9).
Conclusions
Small tumor biopsies are not always sufficient to differentiate SCLC from TC because cells can be altered by crush artifacts. Immunohistochemistry, especially Ki67 staining, seems indispensable to avoid aggressive treatment of minimally proliferating TC with devastating effects on patient outcomes. Careful evaluation of the unique clinical presentation of each patient is necessary and, when in doubt, it is better to revise the histological and immunohistochemical examination.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Detterbeck FC. Clinical presentation and evaluation of neuroendocrine tumors of the lung. Thorac Surg Clin 2014;24:267-76. [Crossref] [PubMed]
- Gustafsson BI, Kidd M, Chan A, et al. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5-21. [Crossref] [PubMed]
- Travis WD. Pathology and diagnosis of neuroendocrine tumors: lung neuroendocrine. Thorac Surg Clin 2014;24:257-66. [Crossref] [PubMed]
- Renshaw AA, Haja J, Lozano RL, et al. Distinguishing carcinoid tumor from small cell carcinoma of the lung: correlating cytologic features and performance in the College of American Pathologists Non-Gynecologic Cytology Program. Arch Pathol Lab Med 2005;129:614-8. [PubMed]
- Pelosi G, Rindi G, Travis WD, et al. Ki-67 antigen in lung neuroendocrine tumors: unraveling a role in clinical practice. J Thorac Oncol 2014;9:273-84. [Crossref] [PubMed]
- Pelosi G, Rodriguez J, Viale G, et al. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol 2005;29:179-87. [Crossref] [PubMed]
- Chong CR, Wirth LJ, Nishino M, et al. Chemotherapy for locally advanced and metastatic pulmonary carcinoid tumors. Lung Cancer 2014;86:241-6. [Crossref] [PubMed]
- Travis WD. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol 2012;25 Suppl 1:S18-30. [Crossref] [PubMed]
- Forde PM, Hooker CM, Boikos SA, et al. Systemic therapy, clinical outcomes, and overall survival in locally advanced or metastatic pulmonary carcinoid: a brief report. J Thorac Oncol 2014;9:414-8. [Crossref] [PubMed]


