Uniportal video-assisted thoracic surgery colorectal lung metastasectomy in non-intubated anesthesia
Introduction
Surgery of lung metastases is still a consolidated curative therapy in selected patients (1,2). Video-assisted thoracic surgery (VATS) are frequently performed to remove these lesions although the impossibility of performing real manual palpation of such masses increases the risk of missing little or deeply located lesions (3,4). For these reasons we introduced an effective hybrid procedure that combines VATS with hand insertion through a transxiphoid approach (3).
Fifteen years ago we started a program of VATS under thoracic epidural anesthesia in awake patients (5) and this—to our knowledge—is the oldest surgical program specifically created for this purpose. We used this approach for many pathologies and namely we successfully performed lung metastasectomy in selected patients (6). This procedure coupled the advantages of minimal surgical invasiveness with the lesser anesthesiological impact thus achieving better results in both short and long terms compared to classic techniques. Nevertheless, the better knowledge of physiopathology, the development of new drugs and the increased confidence with a “breathing” operative field, allowed us to carry out some thoracic procedures using only local anesthesia. Lung metastasectomy was one of these (7). In this study we investigated the feasibility, safety, and efficacy of this combined surgical-anesthesiological technique.
Methods
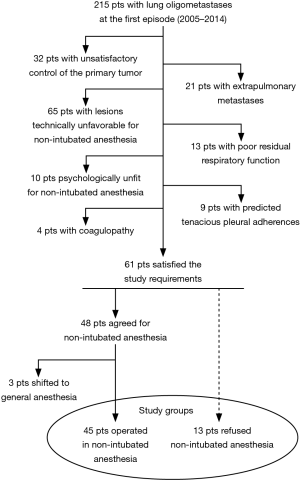
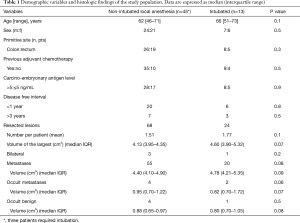
Between December 2005 and December 2014, 48 patients (25 men and 23 women) with lung colorectal oligometastases at the first episode successfully underwent VATS metastasectomy under non-intubated local anesthesia. Patients’ demographic and clinical data about these patients are summarized in Table 1. The study was designed as retrospective and approved by the ethics committee of our university (No. 626/15). All patients released their fully-informed written consent once they were informed about the difference with standard surgical approaches, and the possibility of switching to general anesthesia with one-lung ventilation during the procedure. In the same period 13 patients scheduled for non-intubated metastasectomy refused awake surgery (Figure 1). These patients were then used as control group. Another 3 patients approached with the non-intubated approach required the conversion to general anesthesia for intolerance or unforeseen technical difficulty during the operation and thus were ruled out from statistical evaluation. We excluded from intubated surgery patients with pCO2 greater than 50 mmHg.
Full table
Indications for study inclusion
All patients suitable for the procedure were recruited among those satisfying the classic prerequisites for lung metastasectomy: complete control of the primary tumor, absence of extrapulmonary metastases and enough postresectional respiratory capacity (8). Thereafter other general criteria were considered for VATS metastasectomy: radiologic and at times also echographic absence of pleural adhesion, oligometastases with possible long disease free interval, peripheral location requiring only instrumental palpation, small size entailing no more than 3 cm at computed tomography (CT), lesions removable with a wedge resection.
Supplementary requisites for thoracic surgery in local anesthesia were stable psychological profile with cooperative mood, normal coagulation tests and absence of other bleeding disorders. Patients who refused the procedure in non-intubated modality were used as control group.
Preoperative study
The presence of lung metastases was always diagnosed with CT imaging in the oncological follow-up. After the discovery of the lung lesion, all patients underwent further examination by positron emission tomography (PET) to exclude primary recurrence or extrapulmonary relapse. Before the planned surgery they underwent routine spirometry and arterial blood gases for the evaluation of postoperative pulmonary function. Psychological profile needed to tolerate non-intubated anesthesia was always investigated from a dedicated specialist with dedicated tests and with an interview (9,10).
Anesthesiology
During the surgical procedure patients were continuously monitored: with electrocardiogram, pulse oxymeter, systemic and central venous blood pressure, body temperature, arterial blood gases, and end-tidal CO2 by insertion of one detector into a nostril. All patients underwent the bispectral index intraoperative monitoring (11). This is an objective monitoring modality evaluates the effect of sedative drugs on the central nervous system. It uses processed electroencephalogram signals to measure sedation depth on a unitless scale from 0 to 100 (0, coma; 40 to 60, general anesthesia; 60 to 90, sedated; 100, awake).
An aerosolized 5 mL solution of 2% lidocaine was administered for 5 minutes in order to avoid cough reflex. During the procedure patients breathed O2 through a ventimask to keep oxygen saturation greater than 90%. Intercostal bloc was usually provided by separate local injection of lidocaine 2% (4 mg/kg) and ropivacaine 7.5% (2 mg/kg). Double drug dose was used to achieve a rapid onset with a long duration of the analgesic effect. Internal maneuvers are usually well tolerated with intraoperative intravenous administration of benzodiazepine (midazolam 0.03–0.1 mg/kg) or opioids (remifentanil 15 µg/kg/min). Whenever anxiety or a panic attack occurred perioperatively, sedation was slightly increased by the continuous infusion of propofol (0.5 mg/kg) while maintaining spontaneous breathing. During the procedure patients received Ringer’s lactate solution, which was stopped immediately postoperatively.
Surgical technique
During the surgical procedure is advisable for the surgeon to talk to the patient to test the status of analgesia and to inform him/her about the procedure. Low-volume classic music was played in the operating room. Patients were preventively informed about the discomfort arising with the open pneumothorax, patients were also asked to keep a normal breath rate and supported in this manoeuvre if needed. The operation is performed in lateral decubitus position, usually through a single small 30–40 mm uniport skin incision sited on the most suitable space to reach and remove the suspect metastasis. Rib spreading retractor is always avoided, whereas only Alexis (Alexis®, Applied Medical, USA) wound protector and muscle retractor is allowed. This incision allows the introduction of the operative thoracoscope, the stapler and, when needed, a gauze pad mounted on a ring-forceps to contrast lung movements during breathing.
Digital and instrumental-palpation approaches were always performed to find the lesion. This step is favored by the open pneumothorax, which reduces lung tissue density and facilitates the identification of even smaller nodules.
Finally one 28 Ch chest tube is positioned at the posterior end of the surgical incision. This drainage is kept on the operatory table already connected with a water seal system, thus ready to be inserted into the chest in order to contrast the discomfort from the open pneumothorax. No trans-intercostal suture is required. Muscle sutures are tightened, to obtain maximal lung re-expansion, asking the patient to breathe deeply or to cough; otherwise, this process can be accelerated with air aspiration inside the pleural cavity. Bilateral lesions are approached in two separated sessions in different days.
Drinking, eating and walking were usually restored in the same day of surgery. Patients were discharged after radiological confirmation of complete lung re-expansion and limited production of pleural fluid (no more than 100 mL/day) and absence of air leakage. In the case of prolonged air leak discharge is allowed with Heimlich valve.
Outcome measurements
Intraoperative assessment outlined the timed quantification of ratio of arterial oxygen tension to fraction of inspired oxygen (PaO2/FiO2) and arterial carbon dioxide tension (PaCO2).
State of consciousness and postoperative recovery was evaluated by the Quality of Recovery (QoR-40), which is a 40-item self-administered questionnaire (12). Each item is linked to a 5-point Likert scale [1–5] with a minimum cumulative score of 40 (global maximal impairment) and maximum of 200 (no impairment).
Intra and postoperative pain was assessed using a visual analogue scale (VAS) that graded from 0 (no pain) to 10 (most severe imaginable pain). Patients were asked to grade perceived pain on a graded ruler (13).
Outcome measurements were global in-operating room time, operative time, hospital stay and morbidity.
The impact of the procedure was assessed by titration of serum interleukins 6 and 10, neutrophil/lymphocytes ratio and levels of natural killer lymphocytes. These parameters were determined before the operation and then after 1, 7 and 14 days. Long term results were also considered with disease free survival time after metastasectomy and overall survival.
Statistics
Descriptive statistics was presented as median and interquartile range. All comparisons were performed with the nonparametric test. Comparison was done with patients who refused non-intubated metastasectomy from the very beginning of our study. Survival was calculated by the Kaplan-Meier method. Significant threshold was considered P<0.05. All calculations were made with the SPSS 18 computer software package (SPSS® 18 version, Chicago, IL, USA).
Results
Selection flowchart is schematized in Figure 1. Perioperative and postoperative results in each group are illustrated in Table 2. In particular, the non-intubated group demonstrated a significant difference in global in-operating room time, compared to the intubated group (66 vs. 78 min, P=0.04).

Full table
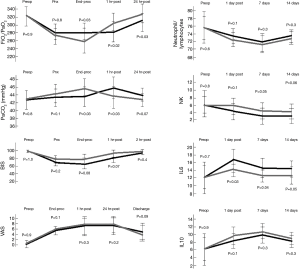
Intubation was decided in three patients on the bases of inability to sustain prolonged pneumothorax thus representing a failure rate of 6.2%. Figure 2 shows the changes of the main intraoperative measurements. As expected, PaO2/FiO2 resulted significantly lower and accordingly PaCO2 significantly higher in the non-intubated patients at the end of the procedure. However, the values inverted at 1 hour postoperatively with a significant difference of PaO2/FiO2 and PaCO2 in favor of the non-intubated group. At 24 hours from the procedure PaO2/FiO2 persisted significantly higher in the non-intubated group. Similarly, QoR40 was found to be significantly higher in the non-intubated group at both 24 and 48 hours from the procedure. No patient manifested a clear complain against the non-intubated procedure and many expressed the desire to be operated in future using the same anesthesia.
A total of 68 nodules were palpated and resected (17 patients had resection of 2 nodules), of which 60 of which had been predicted by CT scan. Mean number of lesions resected per patient were 1.51 (non-intubated) and this was not significantly different from those resected in intubated patients (n=24, mean 1.77, P=0.1) (Table 1). The ratio between lesion expected at CT and found intraoperatively was 1.133. Among the resected nodules 55 were defined metastatic at histologic examination, 4 of which not detected at CT. The median volume of the histology-proven metastatic nodules was 4.40 (4.10–4.90) (Table 1). Non neoplastic nodules were 13 (19%), 4 of which not detected from previously performed CT scan: anthracotic lymph node (n=5), granuloma (n=4) and hamartoma (n=4).
The variations of neutrophil/lymphocyte ratio, lymphocyte subpopulations (natural killers) and interleukins in the postoperative period are illustrated in Figure 2. Notably no difference in lymphocyte count in the seven postoperative days was found between groups, whereas natural killers were significantly more decreased in the intubated group.
Interleukin 6 appeared increased on the first postoperative day with a significant higher value in the intubated group. No differences were found in interleukin 10 levels.
There was no mortality in these groups. Major morbidity rate was higher in the intubated group 3 (6%) vs. 3 (23%) yet not significant (P=0.11). In the non-intubated group we experienced only two patients with persistent air leak and one with arrhythmia, whereas in the control group two developed pneumonia and one had a persistent air leakage. The median hospital stay was 3.0 vs. 3.7 days (P=0.033), but even excluding the hospital stay the estimated costs for the non-intubated procedures were significantly lower.
Thirty-six patients recurred within a median time of 15 months: ipsilateral lung metastases developed in 12 patients and contralateral in 24. Procedures were accomplished again through VATS in majority of the patients, even on the same side of previous surgery.
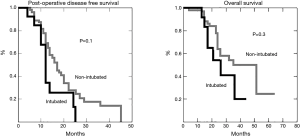
Nineteen patients died of recurrence whereas 26 are still alive 11 of whom clinically tumor free. Both post-operative disease free survival and overall survival (Figure 3) are comparable to those achieved with VATS procedure performed under general anesthesia.
Discussion
In January 2001 a thoracic surgeon in our center (TCM) started a structured program of thoracic surgery performed in thoracic epidural anesthesia in awake patients (14). This program was then approved by the Research Ethics Board of our academic Institution. This native and unique program defined “thoracic surgery in non-intubated anesthesia” is currently carried out by another colleague (VA), from the same center, as main investigator. Indeed an increasing number of pathologies could be approached with this combined surgical-anesthesiological technique.
In this study we demonstrated that VATS resection of colorectal pulmonary oligometastases in non-intubated anesthesia is feasible, safe and effective. The major point against thoracic surgery under non-intubated anesthesia is the feasibility of lung metastasectomy. Indeed, criticism was raised against the technical difficulties originated by an operation conducted in an exiguous and moving operative field (15). In this series we were able to remove all scheduled lesions without need of intubation. The only three cases in which conversion to general anesthesia was required, were due to patient’s incapability to sustain in prolonged pneumothorax. We think that non-intubated operation should be always restricted to selected cases as demonstrated in our accurate selection (Figure 1), which excluded from this technique approximately 80% of the patients initially evaluated.
This procedure was safe, as confirmed by the low morbidity rate compared to both thoracic epidural and general anesthesia. In particular, the operation under spontaneous breathing avoided the adverse effects related to single-lung ventilation resulting in a more physiologic postoperative lung re-expansion. The evaluation of vital parameters demonstrated a satisfactory arterial oxygenation during the operation and particularly during the immediate postoperative hours. This allowed an immediate resumption of many daily activities and a faster recovery and shorter hospitalization. Apart from economical impact of the prolonged hospital stay, the reduced overall time spent in the operating room and the cutback on anesthesiological devices, led to minor costs for the institution.
A depression of immunologic status during the immediate postoperative period was detected in those patients who underwent local anesthesia and considered a collateral finding. This behavior was already reported in our experience (16) and might influence the postsurgical infections thus explaining the lower morbidity rate but it may also have a tumor control effect in these metastatic patients.
This last intriguing perspective may evoke the question about the efficacy of the operation in non-intubated anesthesia. Indeed, less accurate palpation due to the VATS approach, especially if conducted under non-intubated modality, was largely indicated as inadequate with respect to open or hand-assisted approaches (17). However, the refinement of CT scan technology and the accurate selection of the patients with oligometastases might have reduced the risk of undetected lesions. As a matter of fact, survival was not affected by the theoretical limitations of the procedure. On the contrary, despite the hypothetical possibility of missing small lung metastases, we interestingly experienced a longer—yet not significant—disease free survival in this cohort of patients. Due to the relatively small sample size this data has a limited scientific value, but a possible role of cellular-mediate immunology, which resulted less depressed after non-intubated operation (18), deserves a more accurate investigation in the next future.
The confidence acquired with this technique allowed us to perform non-intubated operations also in the case of redo metastasectomy (19,20), which initially were not considered among the indications. The evolution of the instruments also permitted to perform non-intubated lung metastasectomy through a unique transxiphoid incision (7), which proved to be the least painful incision site.
Limitations and criticism
The retrospective study design and the small cohort of patients spread over a long span may be associated with some criticisms, which should be mitigated by the observational nature of the investigation and by the careful patient selection as documented by the Figure 1.
Conclusions
In this study, VATS lung metastasectomy in non-intubated local anesthesia was easily and safely performed in selected patients with oligometastases without affecting long term results. The advantages were significant in overall operative time and hospital stay thus resulting in a better patient satisfaction and lesser economical costs. We believe that further investigation is warranted to confirm our preliminary findings and to extend this surgical-anesthesiological approach also to other pathologies.
Acknowledgements
This research was supported by the Italian Health Ministry (title of the project: ‘Profilo genetico associato al fenotipo metastatico e alla prognosi nei tumori polmonari’).
Professor Vincenzo Ambrogi feels grateful that Professor Tommaso Claudio Mineo, pioneer of Thoracic Surgery at the Tor Vergata University, allowed him to use his personal series of patients. The authors thank all the members of the Official Awake Thoracic Surgery Research Group and those of the Multidisciplinary Lung Metastases Group Tor Vergata University.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was designed as retrospective and approved by the ethics committee of our university (No. 626/15). All patients released their fully-informed written consent once they were informed about the difference with standard surgical approaches, and the possibility of switching to general anesthesia with one-lung ventilation during the procedure.
References
- Perentes JY, Krueger T, Lovis A, et al. Thoracoscopic resection of pulmonary metastasis: current practice and results. Crit Rev Oncol Hematol 2015;95:105-13. [Crossref] [PubMed]
- Abdelnour-Berchtold E, Perentes JY, Ris HB, et al. Survival and Local Recurrence After Video-Assisted Thoracoscopic Lung Metastasectomy. World J Surg 2016;40:373-9. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Ambrogi V, et al. Video-assisted approach for transxiphoid bilateral lung metastasectomy. Ann Thorac Surg 1999;67:1808-10. [Crossref] [PubMed]
- McCormack PM, Bains MS, Begg CB, et al. Role of video-assisted thoracic surgery in the treatment of pulmonary metastases: results of a prospective trial. Ann Thorac Surg 1996;62:213-6; discussion 216-7. [Crossref] [PubMed]
- Mineo TC. Epidural anesthesia in awake thoracic surgery. Eur J Cardiothorac Surg 2007;32:13-9. [Crossref] [PubMed]
- Pompeo E, Mineo TC. Awake pulmonary metastasectomy. J Thorac Cardiovasc Surg 2007;133:960-6. [Crossref] [PubMed]
- Mineo TC, Ambrogi V. Lung metastasectomy: an experience-based therapeutic option. Ann Transl Med 2015;3:194. [PubMed]
- Ehrenhaft JL, Lawrence MS, Sensenig DM. Pulmonary resections for metastatic lesions. AMA Arch Surg 1958;77:606-12. [Crossref] [PubMed]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services, 1971.
- Marks IM, Mathews AM. Brief standard self-rating for phobic patients. Behav Res Ther 1979;17:263-7. [Crossref] [PubMed]
- Kissin I. Depth of anesthesia and bispectral index monitoring. Anesth Analg 2000;90:1114-7. [Crossref] [PubMed]
- Myles PS, Weitkamp B, Jones K, et al. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth 2000;84:11-5. [Crossref] [PubMed]
- Price DD, McGrath PA, Rafii A, et al. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983;17:45-56. [Crossref] [PubMed]
- Mineo TC, Ambrogi V. Efficacy of awake thoracic surgery. J Thorac Cardiovasc Surg 2012;143:249-50; author reply 250-1. [Crossref] [PubMed]
- Mineo TC, Tacconi F. Role of systemic inflammation scores in pulmonary metastasectomy for colorectal cancer. Thorac Cancer 2014;5:431-7. [Crossref] [PubMed]
- Nakanishi R, Yasuda M. Awake thoracoscopic surgery under epidural anesthesia: is it really safe? Chin J Cancer Res 2014;26:368-70. [PubMed]
- McCormack PM, Ginsberg KB, Bains MS, et al. Accuracy of lung imaging in metastases with implications for the role of thoracoscopy. Ann Thorac Surg 1993;56:863-5; discussion 865-6. [Crossref] [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Tacconi F, et al. Multi-reoperations for lung metastases. Future Oncol 2015;11:37-41. [Crossref] [PubMed]
- Treasure T, Mineo T, Ambrogi V, et al. Survival is higher after repeat lung metastasectomy than after a first metastasectomy: Too good to be true? J Thorac Cardiovasc Surg 2015;149:1249-52. [Crossref] [PubMed]