Percutaneous paravalvular leak closure after CoreValve transcatheter aortic valve implantation using an arterio-arterial loop
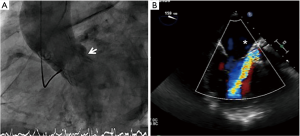
An 84-year-old male was admitted to our Institution due to symptoms and signs of heart failure. Diagnostic work-up revealed the presence of a severe aortic stenosis with moderately impaired left ventricular function (LVF 35%). He was scheduled for transcatheter aortic valve implantation (TAVI), since the patient was considered at a high surgical risk. The patient received a 29-mm CoreValve (Medtronic Inc., Minneapolis, USA) through transfemoral route. Due to significant paravalvular regurgitation (PVR) the valve was post-dilated with a 25-mm Nucleus balloon (NuMED, NY, USA) with the final result of moderate (2+) PVR. The course was uneventful after the procedure and the patient was discharged home. However, at 3-month follow-up the patient complained of symptoms of heart failure. A transthoracic echocardiogram revealed the presence of severe PVR and a severely impaired LVF (20%). Transesophageal echo (TEE) and invasive angiogram showed a severe crescent-shape PVR of 10 mm width located in the posterior aspect of the TAVI frame with patent coronary arteries (Figures 1-3). After discussion with the heart team the patient was considered a candidate for transcatheter paravalvular leak (PVL) closure.


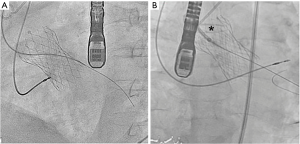
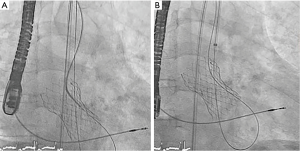
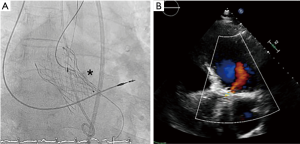
Under general anesthesia and TEE control two 6-Fr sheaths were placed in the right radial (for control aortography) and left femoral arteries (for procedure). Through femoral route a 6-Fr AL1 diagnostic catheter (Cordis, USA) and a 0.0035’ straight Terumo wire were used to probe the defect (Figure 4A). The leak could be crossed and 4 Fr Terumo Glidecath diagnostic catheter (Terumo Medical Corporation, USA) was placed in the LV cavity. Terumo wire was exchanged by a Confida wire (Medtronic Inc., Minneapolis, USA) in order to gain support to advance a 7-Fr TorqVue sheath for AVP-III device insertion (St Jude Medical, USA; selected based upon the measurements of TEE). However, after several attempts the sheath could not be advanced to the LV due to the severe annulus calcification (Figure 4B). Then, the defect was crossed with the AL1, but this time through the radial access. Once the wire was advanced to the LV, it was manipulated into the LV to direct the tip to the left ventricular outflow tract (LVOT) and cross the TAVI leaflets to reach the ascending aorta. Since we had our 7 Fr TorqVue sheath located in that position we were able to directly insert the Terumo wire inside, thus creating an arterio-arterial loop (Figures 5,6). The TorqVue sheath was kept in place to increase the support and a 7.5-Fr JR4 Sheathless catheter (Asahi, Japan) inserted through radial artery was advanced into de LV. Then a 10 mm × 4 mm AVP-III was positioned into the LV. We firstly unsheathed completely the device in order to orientate it with its long axis parallel to the major defect diameter (Figure 7). Once the occluder was properly oriented it was partially recaptured to be positioned into de defect. Eventually the device was implanted with significant reduction of the PVR to mild degree (Figures 8,9). A buddy-wire was left into de LV in case a second occluder was required. However, echo, angiogram and hemodynamic measurements confirmed the excellent result of the case, so no more devices were needed (Figures 10-12).





PVR after TAVI has been linked to an impaired survival (8). The management of paravalvular leaks after TAVI is still a matter of controversy. Mild degrees of PVR (<2/4) might be clinically followed as they are thought to be benign and not progressive in the majority of patients (9). However, more severe degrees of AR may be clinically relevant and deserve intervention. Usually, balloon post-dilatation is the first option for paravalvular leaks following TAVI, using a slightly oversized balloon. In our case, balloon dilatation was the first-line intervention, but failed to solve the PVR. The possibility of implanting a valve-in-valve has also been described (10), with an excellent result. However, this strategy may be of limited value if the leak is produced by the presence of bulky calcification of the annulus. And finally, the use of occlusion devices may be an appropriate technique to deal with such paravalvular leaks. Percutaneous closure of PVL has been described as a safe and effective strategy to reduce the regurgitant volume and improve patients’ symptoms (11,12). Most of the cases reported are treated using the AVP-IV that can be delivered through small diagnostic catheters (11). However, when the leak is big enough, large sheaths may be necessary to implant the appropriate device. In self-expanding prostheses the presence of the valve struts and the annulus calcification may offer high resistance to the advancement of delivery systems mainly when using large sheaths. Arterio-venous loop is frequently used in PVL closure in surgical mitral prosthesis. Notwithstanding, it requires transseptal puncture, place the wire into the left atrium and snare it, therefore increasing the complexity of the procedure. Nevertheless, in our opinion the arterio-arterial loop is easier to create since both arterial sheaths were previously in place. The management of the wire in the LV to achieve the ascending aorta could be challenging, as it is the insertion of the wire in the sheath to create the loop. When direct insertion is not possible, the wire should be snared to be externalized through the arterial access. Once the loop is performed the support to advance large sheaths is significantly increased as it was demonstrated in our case, and the occluder device could be successfully deployed.
Acknowledgements
The patient gave written informed consent to take part in the report.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Estévez-Loureiro R, Benito-González T, Gualis J, et al. Initial aortogram showing severe PVR. Asvide 2017;4:070. Available online: http://www.asvide.com/articles/1377
- Estévez-Loureiro R, Benito-González T, Gualis J, et al. X-plane TEE showing the severe paravalvular regurgitation. Asvide 2017;4:071. Available online: http://www.asvide.com/articles/1378
- Estévez-Loureiro R, Benito-González T, Gualis J, et al. Creation of the arterio-arterial loop. Asvide 2017;4:072. Available online: http://www.asvide.com/articles/1379
- Estévez-Loureiro R, Benito-González T, Gualis J, et al. AVP-III oriented into LV. Asvide 2017;4:073. Available online: http://www.asvide.com/articles/1380
- Estévez-Loureiro R, Benito-González T, Gualis J, et al. AVP-III in place through the defect. Asvide 2017;4:074. Available online: http://www.asvide.com/articles/1381
- Estévez-Loureiro R, Benito-González T, Gualis J, et al. Aortogram showing almost completely closure of the defect. Asvide 2017;4:075. Available online: http://www.asvide.com/articles/1382
- Estévez-Loureiro R, Benito-González T, Gualis J, et al. TTE with mild residual aortic regurgitation. Asvide 2017;4:076. Available online: http://www.asvide.com/articles/1383
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95. [Crossref] [PubMed]
- Rallidis LS, Moyssakis IE, Ikonomidis I, et al. Natural history of early aortic paraprosthetic regurgitation: a five-year follow-up. Am Heart J 1999;138:351-7. [Crossref] [PubMed]
- Rodés-Cabau J, Dumont E, Doyle D. "Valve-in-valve" for the treatment of paravalvular leaks following transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2009;74:1116-9. [Crossref] [PubMed]
- Gafoor S, Franke J, Piayda K, et al. Paravalvular leak closure after transcatheter aortic valve replacement with a self-expanding prosthesis. Catheter Cardiovasc Interv 2014;84:147-54. [Crossref] [PubMed]
- Estévez-Loureiro R, Salgado-Fernández J, Vázquez-González N. Percutaneous closure of paravalvular leaks after transcatheter aortic valve implantation with Edwards SAPIEN prosthesis: a report of two cases. J Invasive Cardiol 2013;25:92-5. [PubMed]


