Lower expression of platelet derived growth factor is associated with better overall survival rate of patients with idiopathic nonspecific interstitial pneumonia
Introduction
Idiopathic nonspecific interstitial pneumonia (INSIP) is generally characterized by hyperplastic type II pneumocytes with inflammatory cell infiltration, alveolar septum uniformly broadening with or without fibrosis, collagen deposition, occasional fibroblastic foci and honeycomb appearance of the lung (1,2) . INSIP is a major sub-type of idiopathic interstitial pneumonias (IIPs), which is a diverse group of lung disorders of unknown etiology characterized by various degrees of alveolar inflammation and remodeled alveolar structure that often result in pulmonary fibrosis (3,4). INSIP and idiopathic pulmonary fibrosis (IPF) share similar histomorphology, the latter also as the main sub-type of IIPs, and typically exhibits chronic fibrosis interstitial pneumonia, fibroblastic foci, and honeycomb changes (5), even though these two diseases were thought have etiological and inheritance heterogeneity. At present, many patients with IIPs respond to corticosteroid therapy to a certain degrees, but few achieve completely remission. Therefore, more effect needs to explore new potential strategy treatment for patients with IIPs, including INSIP and IPF.
Previous studies have suggested that many cytokines, including interleukins (ILs), transforming growth factor-beta (TGF-β), alpha-smooth muscle actin (α-SMA), and BMP-7 have pro-fibrogenic effects (6-9). Since patients with IPF frequently exhibit fibrotic lesion and have poor prognosis, many studies have focused on the role of these cytokines in IPF, and few investigations uncover the aberrantly expressed cytokines involved in the pathogenesis of patients with INSIP. In addition to, the clinical outcome of abnormally expressed cytokines in patients with INSIP is also still unclear.
In this study, we hypothesized that various cytokines were abnormally produced in the patients with INSIP. We determined the expression profile of cytokines in INSIP, including IPF by Oligo GEArray. Our initial results were validated by tissue-array with immunohistochemistry analysis and real-time PCR. Finally, the clinical outcome of related cytokines was further analyzed in 22 cases of INSIP and 25 cases of IPF. Our study aimed to identify the involvement of critical cytokines in the advancement of INSIP, and clarify whether these cytokines are related with the survival rate of patients with INSIP and IPF.
Methods
Human subjects
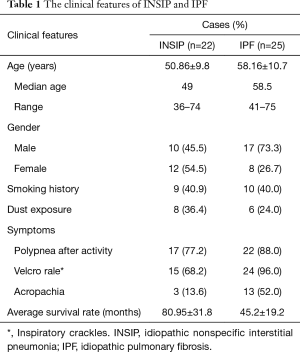
This study was approved by the ethics committee of Tongji Hospital [(Tong) No. 183 Ethics]. All samples were collected from patients with biopsy-confirmed INSIP and IPF from year 1999 to 2009, and all patients provided informed consent. Criteria for inclusion of subjects: (I) Biopsies for Oligo GEArray taken by video-assisted thoracoscope surgery or small incision lung biopsy. The control biopsy samples were collected from normal tissues of benign pulmonary tumors. Specimens used for tissue array and immunohistochemistry included 25 cases of IPF and 22 cases of INSIP (4 cases are cellular type, 18 cases are fibrosing type), which were collected at the time of diagnostic surgical lung biopsy through Tongji Hospital and Shanghai Pulmonary Hospital, affiliated with Tongji University School of Medicine; (II) the criteria of diagnosis referred to the American Thoracic Society (ATS)/European Respiratory Society (ERS) classification guidelines on IIP in 2002 (2), ATS/ERS views on INSIP Classification and Diagnostic Criteria in 2008 (10), Guidelines for Diagnosis and Management of IPF in 2011 (11) and Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias in 2013 (12); (III) the final diagnosis involved in multiple-disciplinary discussion is mutually made by pathologists, clinician and radiologists, and except other known causes of interstitial lung disease (ILD); (IV) each case had integrated clinical, radiologic, and pathologic data, including at least the follow-up data of more than 5 years. Besides, all patients received Glucocorticoids treatment, the Glucocorticoid use as following: (I) a large dosage: 100–200 mg/d methylprednisolone via intravenous injection, then 40 mg/d per os after 10 days, the dosage of methylprednisolone could be reduce until 4 weeks; (II) ordinary dosage: 0.5 mg/kg prednisone via per os, 0.25 mg/kg per os after 4 weeks, 0.125 mg/kg per os after 8 weeks or 0.25 mg/kg per os q.o.d (13). The clinical information of all patients included in the study is shown in Table 1.
Full table
Oligo GEArray
To monitor the expression profile of cytokines in patients with INSIP, including IPF, Oligo GEArray was employed. We extracted total RNA from human samples (3 cases of IPF and INSIP, and 1 case of normal control) that were grown on plastic plates, using the RNA Stat-60 reagent, and converted RNA into biotin-labeled cRNA target probes for microarray hybridization using the True Labeling-AMP linear RNA amplification kit. The cRNA targets (2 µg of cRNA) were next hybridized with oligonucleotide probes, representing different cytokines, printed on a nylon membrane. The resulting products on arrayed membranes were detected by a chemiluminescent detection kit, and analyzed by GEArray Analyzer data analysis software.
Tissue array design and immunohistochemistry (IHC)
Before constructing tissue array (TMA), typical lesions of 25 cases of IPF and 22 cases of INSIP were evaluated under light microscope. Then the TMA was performed using a core diameter of 2 mm by Shanghai Outdo Biotech laboratory. Each slide contained 77 lesion cores and 1 normal tissue core. IHC of paraffin-embedded sections was carried out using a standard streptavidin-biotinylated alkaline phosphatase (ABC-AP, DakoCytomation, Hamburg, Germany) method. The following antibodies were used: TGF-β1 (Santa Cruz, 1:100), fibroblast growth factor 10 (FGF10) (Santa Cruz, 1:100), platelet derived growth factor (PDGF) (Santa Cruz, 1:100). Under low-power magnification (100×), positive staining cells were screened and images of five representative fields were then captured under high-power magnification (400×) in Leica DMLA light microscope (Leica Microsystems, Wetzlar, Germany). The positive cell density of each core was counted by two independent investigators blind to clinical outcome and knowledge of the clinicopathological data. Data were expressed as mean value (± SE) of the triplicate cores taken from each patient.
Quantitative real-time reverse transcription polymerase chain reaction (QRT-PCR)
Total RNA was extracted from 50 mg biopsy using Trizol plus kit (TaKaRa, Japan). First-strand cDNA synthesis was done using Promega kit. Synthesized cDNA was used for QRT-PCR analysis using LightCycler (Roche, Switzerland) following the manufacturer’s instructions. TGF-β1, FGF10, and PDGF primers were specifically designed by Biosune Bio-Technology Co., Ltd (China). β-actin was used as the internal control. The Nucleotide sequences for primers: TGF-β1: 5'-CGACTACTACGCCAAGGAG-3', 5'-GAGAGCAACACGGGTTCAG-3'; FGF10: 5'-AGAGCGACCCTCACATCAAG-3', 5'-TCGTTTCAGTGCCACATACC-3'; PDGF: 5'-CCTGCCCATTCGGAGGAAGAG-3’, 5'-TTGGCCACCTTGACGCGCG-3'; β-actin: 5'-CCTGTACGCCAACACAGTG-3', 5'-ATACTCCTGCTTGCTGATCC-3'. Amplifications were carried out in the 20 µL reaction mixtures in the following conditions 95 °C for 2 min and followed by 40 cycles of 95 °C for 20 s, 55 °C for 30 s and 72 °C for 40 s, and then 72 °C for 5 min. The same experiment was repeated 3 times and similar results were obtained. The relative mRNA expression level was calculated and statistically analyzed using delta-delta-Ct method.
Microarray data analysis
Hierarchical clustering of 127 cytokines were performed using the software CLUSTER 3.0 (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm) and displayed by the software Java TreeView (http://www.yiiframework.com/forum/index.php?/topic/9180-the-tree-view/). For the network analysis, all genes were uploaded into the STRING 9.0 database (Search Tool for the Retrieval of Interacting Genes) to analyze the Protein-Protein interaction (PPI). Based on the neighborhood, gene fusions, co-occurrence, co-expression, experiments, and literature mining, STRING database provides information on both experimental and predicted interactions. In this study, we constructed the PPI network based on confidence score of 0.4, which implied that all possible interactions with low level of confidence were extracted from the database and as many as possible were considered, and we used Cytoscape v2.8.3 software for visual analysis of the constructed networks.
Statistical analysis
All measurement data were expressed as mean ± SD, the difference among groups compared using ANOVA, enumeration data were analyzed by chi-square test. Kaplan-Meier method was employed to evaluate survival curve, and the log-rank test was used to compare survival time among groups. The test results were reported as 2-tailed P values, where P<0.05 was considered to be statistically significance.
Results
Identification of cytokines involved in the pathogenesis of INSIP
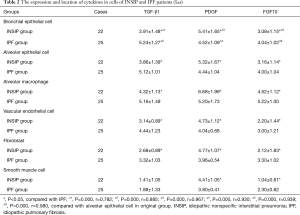
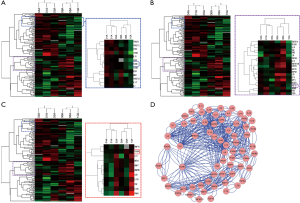
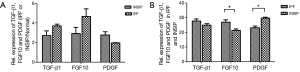
To identify cytokines involved in the pathogenesis of INSIP, with IPF as isotype control, Oligo GEArray that profiled 127 cytokines was employed. The analysis identified 109 cytokines as differentially expressed more than 2-fold with P value <0.05 between normal and lesion tissues. These included cytokines involved in regulation of cell cycle and proliferation, growth factor activity, and protein biosynthesis (Table S1). Specifically, TGF-β1, FGF10, and PDGF were dramatically up-regulated in patients with INSIP (Figure 1A,B,C), and were found closely related with pathogenesis of INSIP (Figure 1D). Similar phenotype was also found in patients with IPF. The quantitative analysis of these cytokines from GEArray is shown in Figure 2A. Interestingly, we found that the expression of TGF-β1 and FGF10 were higher in IPF than in INSIP, while PDGF was expressed at a higher level in patients with INSIP, though there have no statistical significance between INSIP and IPF groups.
Full table
TGF-β1, PDGF, and FGF10 expression were increased in INSIP patients
To confirm above results, we also performed RT-PCR to detect the expression of TGF-β1, PDGF, and FGF10 in patients with INSIP, including IPF. Consistence with results of Oligo GEArray, these genes was obviously increased in patient samples regardless of INSIP and IPF comparing the normal tissue (P<0.05). Their expressions were also individually analyzed in both INSIP and IPF patients. As demonstrated in Figure 2B, PDGF was relatively overexpressed in INSIP, whereas TGF-β1 and FGF10 were highly expressed in IPF (P<0.05), indicating the potential existence of a predominant expression axis of these cytokines in different subtypes of IIPs. Taken together, these results suggested that TGF-β1, FGF10, and PDGF were abnormally expressed in IIPs disease.
The expression and location of TGF-β1, PDGF, and FGF10 in INSIP by IHC detection
Since we observed dramatically increased expression of TGF-β1, PDGF, and FGF10 in both INSIP and IPF at the transcriptional level, we analyzed 22 cases of INSIP and 25 cases of IPF to determine the expression of these cytokines by immunohistochemical analysis. Comparing with normal lung tissues, TGF-β1, PDGF, and FGF10 were highly expressed in various type of cells in both INSIP and IPF (Figure 3A-I), including bronchial epithelial cells, alveolar epithelial cells, macrophage in alveolus and its mesenchyme, vascular endothelial cells, fibroblast (FB) and smooth muscle cells. Importantly, we observed that PDGF was more strongly expressed in patients with INSIP than patients with IPF (Figure 3C,F). Of note, the alveolar macrophages (AM) showed stronger expression of these cytokines than other type of cells. Consistently, through quantification of these cytokines, TGF-β1 and FGF10 were highly expressed in IPF than in INSIP, while PDGF was strongly expressed in INSIP (Table 2), which was consistent with the results of GEArray and RT-PCR. Based on these results, we hypothesized that there was likely a priority of the effect of TGF-β1, PDGF, and FGF10 in INSIP and IPF, and PDGF may be the predominant disease-promoting factor in INSIP, while TGF-β1 and FGF10 may be more important for progression of IPF, although they were all highly expressed in both IPF and INSIP.

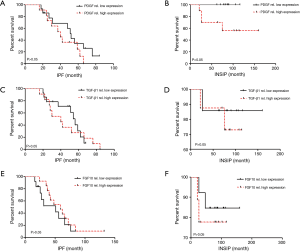
The relationship between PDGF and overall survival rate of patients with INSIP
Due to the relatively high expression of PDGF in patients with INSIP, we wonder whether its expression may be associated with the survival rate of patients. Additionally, the relationship between TGF-β1 and FGF10 expression with the patients’ survival rate were also studied. As mentioned in previously, each case had integrated clinical follow-up data of more than five years. Interestingly, we found that patients with above average PDGF expression survived better than those with PDGF expression below the average level. However its expression levels had no significant correlation with the survival rate of IPF patient (Figure 4A,B). Additionally, PDGF is not an independent prognostic factor of INSIP patients. Besides, our data also showed that TGF-β1 expression also had no significant correlation with patients’ survival rate in INSIP or IPF as well as FGF10 correlation analysis (Figure 4C,D,E,F). Taken together, our data suggests there is significant correlation ship between PDGF expression and survival rate of patients with INSIP.
Discussion
Our results demonstrated that IIPs, including IPF and INSIP, are associated with many abnormally-expressed cytokines. Oligo GEArray identified several cytokines which appeared to be important in the pathogenesis and advancement of INSIP and IPF. In further analysis, we found that TGF-β1, FGF10, and PDGF were dominantly up-regulated in patients with INSIP, as well as IPF. These results were also confirmed by RT-PCR and IHC. Interestingly, TGF-β1 and FGF10 were preferentially increased in IPF than that in INSIP, while PDGF was increasingly expressed in INSIP, indicating there was likely a priority-effect of these cytokines in the progression of IPF and INSIP. Importantly, we found that the negative correlationship between PDGF expression and overall survival rate of patients with INSIP.
Recently, high throughput technique was employed to screen potential therapeutic targets and biomarkers for IIPs. Kaminski et al. described global changes of gene expression in IPF by using the reductionist “cherry picking” and quantitative “systems” approach (6). Yang et al. evaluated transcriptional signatures between sporadic IIPs and familial IIPs, and CXCL12 was identified as a key regulator in the pathogenesis of the disease (8). Similarly, we screened the expression of 127 various cytokines in INSIP by Oligo GEArray, with IPF as isotype control. Expectedly, many cytokines were disorderly expressed in INSIP, including ILs, tumor necrosis factor, osteogenesis protein families and so forth. It is noteworthy that TGF-β1, FGF10, and PDGF were dominantly over-expressed in disease, suggesting they might be disease-drivers of INSIP and IPF. Several studies uncovered TGF-β1 as a well-known pro-fibrogenic factor (7,14-17), and pirfenidone or nintedanib could be used in IPF-involved dysfunction of TGF-β1 (18-21). But little is known about FGF10 and PDGF in the pathogenesis of pulmonary fibrosis and their therapeutic potential in INSIP and IPF, especially as studies have lacked human clinical relevance evidence that may identify the role of these cytokines in IIPs.
To clarify the clinical outcome of TGF-β1, FGF10, and PDGF in INSIP, we used tissue array and IHC to detect their expression in 22 cases of INSIP and 25 cases of IPF subjects. Similar to the report by Gu et al., which identified TGF-β1 and FGF as highly expressed in these two diseases, and localized in alveolar epithelial cells, AM and bronchial epithelium (17), we found that TGF-β1 and FGF10 were strongly expressed in these cells in our subjects. Interestingly, all these cytokines were predominantly expressed in AM, indicating AM are a major resource for the production of these cytokines. Lemaire et al. also found that AM isolated from lung fibrosis in rats induced by asbestos, releases a FGF which persisted over time (22). Importantly, we discovered that PDGF, which was more strongly expressed in INSIP. Intriguingly, some studies indicate that PDGF can promote the proliferation of fibroblasts (23). In bleomycin-induced mice, PDGF was significantly increased in murine pulmonary tissues (24), and target its expression could availably prevent the progress of fibrosis. In human studies, similar results show that imatinib, which specifically inhibits PDGF tyrosine kinase (25), could obviously improve the pulmonary function in IPF patients (26). Accordingly, our study also suggests that PDGF may a potential target for pulmonary fibrosis, especially for INSIP patients since its expression was negatively associated with the survival rate of patients with INSIP in our study. However, further analysis indicates that PDGF is not an independent prognostic factor for INSIP patients. This may due to the relatively small sample size. Thus the value of PDGF in the prognosis of INSIP need verified in more large samples in future studies.
In summary, our study investigated cytokine expression in INSIP and IPF subjects. To the best of our knowledge, most previous studies that have explored the potential therapeutic target or mechanisms of interstitial pneumonia have used cells or animal models, but few studied at the human pathologic level, especially for Asian population. Through analyzing gene expression profiling, we found that the expression of TGF-β1, FGF10, and PDGF were all increased in patients. Importantly, our results suggests that a potential priority effect exists among these cytokines in INSIP and IPF, whereby PDGF may be more important in the pathogenesis of INSIP, whereas TGF-β1 and FGF10 may be more critical for the advancement of IPF. Importantly, our findings suggest that lower expression of PDGF is associated with better overall survival rate of patients with INSIP.
Acknowledgements
Funding: This work was supported by the Science and Technology Commission Foundation of Key Medical Research Foundation of Shanghai, China (034119868; 09411951600), Key Medical Research Foundation of the Health Bureau of Shanghai, China (20134034) and National Nature Science Foundation of China (81570053).
We sincerely thank Yudong Zhang, Jun Gu for their skillful technique support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the ethics committee of Tongji Hospital [(Tong) No. 183 Ethics]. And all patients provided informed consent.
References
- Daniil ZD, Gilchrist FC, Nicholson AG, et al. A histologic pattern of nonspecific interstitial pneumonia is associated with a better prognosis than usual interstitial pneumonia in patients with cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 1999;160:899-905. [Crossref] [PubMed]
- American Thoracic Society. European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [PubMed]
- Swigris JJ, Kuschner WG, Kelsey JL, et al. Idiopathic pulmonary fibrosis: challenges and opportunities for the clinician and investigator. Chest 2005;127:275-83. [PubMed]
- Green FH. Overview of pulmonary fibrosis. Chest 2002;122:334S-339S. [Crossref] [PubMed]
- Fang X, Luo B, Yi X, et al. Usual interstitial pneumonia coexisted with nonspecific interstitial pneumonia, What's the diagnosis? Diagn Pathol 2012;7:167. [Crossref] [PubMed]
- Kaminski N, Rosas IO. Gene expression profiling as a window into idiopathic pulmonary fibrosis pathogenesis: can we identify the right target genes? Proc Am Thorac Soc 2006;3:339-44. [Crossref] [PubMed]
- Murray LA, Chen Q, Kramer MS, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int J Biochem Cell Biol 2011;43:154-62. [Crossref] [PubMed]
- Yang IV, Burch LH, Steele MP, et al. Gene expression profiling of familial and sporadic interstitial pneumonia. Am J Respir Crit Care Med 2007;175:45-54. [Crossref] [PubMed]
- Gu P, Luo B, Yi X, et al. The expressions and meanings of BMP-7 and TGF-β in idiopathic pulmonary fibrosis and idiopathic nonspecific interstitial pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2014;37:664-70. [PubMed]
- Travis WD, Hunninghake G, King TE Jr, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med 2008;177:1338-47. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Wu XY, Yi XH, Li HP, et al. A study on the efficacy of glucocorticoid therapy for idiopathic nonspecific interstitial pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2010;33:593-6. [PubMed]
- Wei X, Xia Y, Li F, et al. Kindlin-2 mediates activation of TGF-β/Smad signaling and renal fibrosis. J Am Soc Nephrol 2013;24:1387-98. [Crossref] [PubMed]
- Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2014;71:549-74. [Crossref] [PubMed]
- Goodwin A, Jenkins G. Role of integrin-mediated TGFbeta activation in the pathogenesis of pulmonary fibrosis. Biochem Soc Trans 2009;37:849-54. [Crossref] [PubMed]
- Gu L, Xu WB, Liu HR, et al. Different cytokine profiles in usual interstitial pneumonia and nonspecific interstitial pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2003;26:350-3. [PubMed]
- Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov 2012;11:790-811. [Crossref] [PubMed]
- Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther 1999;291:367-73. [PubMed]
- Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther 1999;289:211-8. [PubMed]
- Margaritopoulos G. Challenges in IPF diagnosis, current management and future perspectives: Patient case 2. Sarcoidosis Vasc Diffuse Lung Dis 2015;32 Suppl 1:38-9. [PubMed]
- Lemaire I, Beaudoin H, Dubois C. Cytokine regulation of lung fibroblast proliferation. Pulmonary and systemic changes in asbestos-induced pulmonary fibrosis. Am Rev Respir Dis 1986;134:653-8. [PubMed]
- Walsh J, Absher M, Kelley J. Variable expression of platelet-derived growth factor family proteins in acute lung injury. Am J Respir Cell Mol Biol 1993;9:637-44. [Crossref] [PubMed]
- Maeda A, Hiyama K, Yamakido H, et al. Increased expression of platelet-derived growth factor A and insulin-like growth factor-I in BAL cells during the development of bleomycin-induced pulmonary fibrosis in mice. Chest 1996;109:780-6. [Crossref] [PubMed]
- Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 1996;2:561-6. [Crossref] [PubMed]
- Distler JH, Manger B, Spriewald BM, et al. Treatment of pulmonary fibrosis for twenty weeks with imatinib mesylate in a patient with mixed connective tissue disease. Arthritis Rheum 2008;58:2538-42. [Crossref] [PubMed]