Definitive concurrent chemoradiotherapy with S-1 and cisplatin in elderly esophageal squamous cell carcinoma patients
Introduction
Carcinoma of the esophagus is the eighth most commonly diagnosed cancer and the sixth leading cause of cancer death worldwide (1-3). The morbidity is increasing in China and 90% of cases are esophageal squamous cell carcinoma (ESCC) (4). Despite clinical advances in the treatment of esophageal cancer, the 5-year overall survival (OS) rate is poor with no better than 20%. Esophagectomy is the optimal treatment for early staged and localized diseases, but is less frequently performed in elderly patients. Series of reports had demonstrated that postoperative mortality rates in elderly patients ranged from 4.5–23% and may even reach 60% (5-7). Definitive concurrent chemoradiotherapy (dCRT) has also been recommended as the standard non-surgical treatment option for locally advanced esophageal cancer. The seminal Radiation Therapy Oncology Group (RTOG) phase III 85-01 trial compared the efficacy of dCRT based on 5-fluorouracil (5-Fu) and Cisplatin (CDDP) with radiotherapy (RT) alone for thoracic T1-3N0-1M0 esophageal cancer patients, which resulted in a long-term survival rate of 26%, an outcome similar to that with surgery (8). However, the toxicity associated with dCRT was substantial and the severe and fatal side effects were observed in 44% and 20% in the dCRT group comparing to 28% in the RT alone group. Moreover, the efficacy of this combination was about 25–35% and only 23% of the enrolled patients were aged over 70 years, which brought a question about the suitability of dCRT for elderly patients. Subsequently, several studies in the literature demonstrated that elderly esophageal cancer patients might also benefit from dCRT with 5-Fu and CDDP, but the life threatening adverse events were increasing. In a retrospective study which investigated the toxicity of dCRT for aged ≥75 ESCC patients, results showed that treatment-related mortality rate was suspected in up to 18% (9). Thus, exploring new dCRT regimens with better tolerance and lower toxicity for elderly patients are desperately needed.
S-1, a combination of tegafur-gimeracil-oteracil, has been widely used in the clinical. Basic studies showed that S-1 has superior anti-cancer effects than 5-Fu and enhances the sensitivity of cancer cells to the effects of RT (10). Clinical evidence also supports the opinion that dCRT regimen combined CDDP with S-1 represents a viable treatment for esophageal cancer. In studies from East Asian, S-1 plus CDDP concurrent with RT achieved encouraging response rates of 64.4–89.7%. In addition, toxicities associated with this regimen are modest (11-13).
Considering high-grade evidence for the use of S-1 and CDDP combined with RT for elderly ESCC is in shortage, we carried out this retrospective study to investigate the feasibility and efficiency of S-1/CDDP/RT in the setting of dCRT for elderly ESCC patients, focusing on treatment compliance, response rate, toxicity, and survival outcomes.
Methods
Patients
This study was approved by the institutional review board of the First Affiliated Hospital of Soochow University (FAHSU No. 2016067). Written informed consent was obtained from all participants. Between January 2012 and December 2014, 56 elderly ESCC patients who received S-1/CDDP/RT at the cancer center of the FAHSU were screened. Inclusion criteria in our study included: (I) aged 70 years or older at the time of diagnosis; (II) cytopathological confirmed as ESCC; (III) clinical stages according to the 2002 (version 6.0) American Joint Committee on Cancer staging system; (IV) Eastern Cooperative Oncology Group performance status (ECOG PS) 0-1 and were able to intake S-1 as a capsule or as powder with water; (V) no uncontrolled serious diseases and no severe bone marrow, hepatic, renal, respiratory, and cardiac dysfunction. Dysphagia degree was evaluated with the DeMeester symptom scores (14).
Radiotherapy
Radiotherapy was delivered with 6- or 10-Mv linear accelerator. The gross tumor volume (GTV) included the primary tumor and enlarged lymph nodes. The clinical target volume (CTV) was outside expansion of GTV by 5–8 mm, and the boundary was expanded 3–4 cm in the superior and inferior directions. The planning target volume (PTV) was formed by isotropic expansion of CTV by 5–8 mm. The total radiation dose was 5,400 cGy which was given in 27–30 fractions of 1.8–2.0 Gy once-daily fraction for 5 days per week.
Chemotherapy
S-1 was delivered orally twice daily for two weeks at a dose of 70 mg/m2/day (15), and CDDP 75 mg/m2 was administered intravenously in 250 mL of sodium chloride solution on Day 1. This treatment schedule was repeated every 3 weeks. Patients who achieved a response greater than stable disease received additional two cycles of S-1 and CDDP.
Nutritional therapy
Nutritional support including oral nutritional supplements, enteral nutrition, and/or parenteral nutrition was routinely performed in our ward. Patients who developed severe dysphagia during the treatment course had nasogastric tube placement, depending on the treatment week in which this occurred.
Dose modification
The drug dosage was adjusted on a weekly basis. S-1 was reduced to 60 mg/m2/day in the following courses if: grade 3 neutropenia with infection, or grade 4 leucocytopenia, or grade ≥3 thrombocytopenia. Granulocyte colony-stimulating factor (G-CSF) was used to treat for the occurrence of febrile neutropenia. If the creatinine clearance decreased to less than 50 mL/min, the CDDP dose was reduced to 80%. Radiotherapy was suspended for patients with severe esophagitis (grade ≥3), neutropenia (grade 3) with fever, or grade 4 leucocytopenia. Irradiation was restarted when toxicities recovered to grade ≤2.
Evaluation methods
Physician reported toxicities were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0). Clinical response was evaluated according to RECIST (Response Evaluation Criteria in Solid Tumors, version 1.1) system 3 weeks after the completion of treatment. Late toxicity was evaluated based on the RTOG/European Organization for Research and Treatment of Cancer (EORTC) late radiation morbidity scoring scheme six months after the completion of dCRT (16).
Follow-up
Patients were basically followed with physical examination, blood tests, barium swallow X-ray, and enhanced computed tomography (CT) of the chest and abdomen every month in the first half of the year, every 2 months in the second half of the year, every 3 months in the second year and at 6-month interval after two years. Patterns of treatment failure were defined as any sign of recurrent diseases, which could be local-regional, distant, and/or both. When a recurrence was questionable by any of the above examinations, upper gastrointestinal endoscopy (± biopsy) or positron emission tomography/CT (PET/CT) was performed based on the patient’s choice and compared those data with the original CT-based radiation treatment plans.
Statistical analysis
OS was measured from the date of dCRT initiation and the last follow-up or the date of death. Progression-free survival (PFS) was defined as the duration between the date of treatment initiation and the date of documented failure or the date of the last follow-up. Survival curves were determined using the Kaplan-Meier method. Prognostic factors for OS and PFS were obtained using the log-rank test and P value <0.05 was considered statistically significant. All statistical analyses were conducted using Statistical Package for the Social Sciences (SPSS 20.0, Inc., Chicago, IL, USA; Microsoft, Redmond, WA, USA).
Results
Patient characteristics
From January 2012 to December 2014, 56 consecutive elderly ESCC patients who received S-1/CDDP/RT at the cancer center of the FAHSU were screened. The baseline characteristics of the patients were detailed in Table 1. The median age of the 56 patients was 74, ranging from 70 to 87 years old. 46 were male and ten were female. 60.7% patients had a ECOG performance status of 0 and 26.8% had an initial weight loss >10%. The middle thoracic esophagus was the most frequent primary location, occurring in 24 patients (42.9%). The most frequent tumor stage was T3, which was observed in 23 patients. N1 disease was observed in 34 patients. Fifteen patients were diagnosed with M1a.
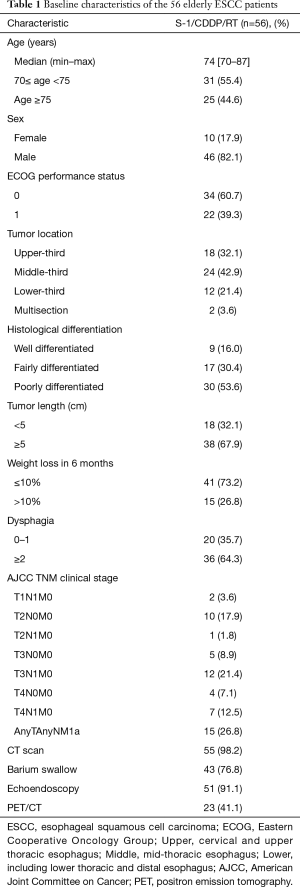
Full table
Treatment tolerance
All patients received the first cycle of S-1 and CDDP with full dose intensity. Four elderly patients refused the second cycle of chemotherapy for the following reasons: one patient developed lung metastasis during treatment, two patients aged over 75 years got fever after developing grade 4 leucocytopenia and one patient had financial problem. All these patients also gave up radiotherapy. Six (10.7%) patients required dose reduction in the second cycle of chemotherapy and the actual dose of S-1 and CDDP was reduced to 60 mg/m2/day and 60 mg/m2, respectively. Among these patients, four were greater than 75 years of age. Planned radiation therapy was completed in 52 of 56 patients, including eight patients with radiation delay for developing grade ≥3 esophagitis. The left four patients gave up radiation therapy for the reasons previous reported. Thus, a total of 38 (67.9%) patients completed dCRT without changing treatment plan.
Acute and late toxicities
The toxicity profile related to the treatment was listed in Table 2. Toxic reactions were assessed in all patients. The most common hematologic toxicities were leucocytopenia. Grade 3 and 4 leucocytopenia were observed in 47 (48.2%) and four (7.1%) patients, respectively. Most patients recovered by using G-CSF. Of these patients, 18 (58.1%) were greater than 75 years of age. A significantly higher incidence of severe leucocytopenia was observed between patients aged ≥75 and 70≤ age <75 years. Neutropenia ranked the second place in the data with the incidence of 68.0% and 42.0% for patients aged ≥75 and 70≤ age <75 years, respectively. An approximately statistical difference was noted for the two subgroups. There were seven (12.5%) patients with grade 3–4 thrombocytopenia and six (10.7%) patients with grade 3–4 anemias. Totally, 41 (73.2%) patients got all grades esophagitis including 8 patients experienced grades 3–4 (seven for grade 3 and one patient for grade 4). Other grade 3 non-hematologic toxicities included anorexia (12.5%), fatigue (10.7%), diarrhea (7.1%) and nausea/vomiting (5.4%). None statistical significance were observed between patients aged ≥75 and 70≤ age <75 years. In terms of late toxicities, 3 (5.4%) patients experienced severe esophageal stenosis and two (3.6%) patients were diagnosed with severe radiation-related pneumonitis. There were no patients died of toxicities during treatment course.
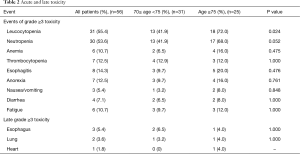
Full table
Response to dCRT and survival outcome
All patients were evaluated for response based on the RECIST system. Complete response (CR) was observed in 30 (53.6%) patients, partial response (PR) in 17 (30.4%) patients, SD in four (7.1%) patients and PD in five (8.9%) patients, yielding an overall objective response rate (ORR) of 84.0%. Moreover, 34 patients (CR in 21 patients, PR in 10 patients and SD in 3 patients) underwent additional esophageal biopsy to confirm the clinical response in our study. Of the 34 patients, tumor cells were not found in 22 patients, only heterocyst cells were found in eight patients, and four patients had persistent disease according to the pathological reports. Only 5 patients (CR in 1 patient, PR in 3 patients and SD in 1 patient) received post treatment PET/CT scan, 18F-FDG uptake decreased to the background level in the only CR patient. In stage I-II and stage III patients, the CR rates were 82.3% and 54.2%, respectively. In stage IVa patients, the CR rate was 20%. The CR rate showed stages I–III to be significantly more common than stage IVa (P=0.002). Improvement of dysphagia by more than one grade was seen in 48 patients.
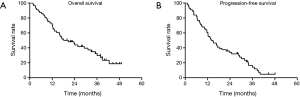
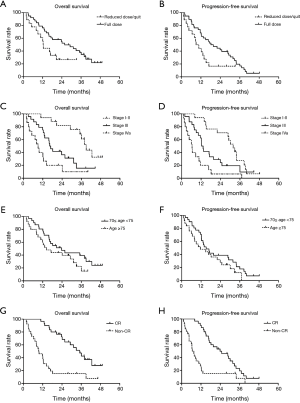
The median duration of follow-up was 21.4 months (range, 1.8 to 49.1 months). At the end of the last follow-up by June 30, 2016, 50 patients experienced treatment failures. The recurrent sites were detailed as follows: 24 for local recurrence, 12 for regional failures, and 11 for distant metastasis. Three patients were considered as for both loco-regional and distant metastasis. The OS and PFS curves for all patients were shown in Figure 1. The median OS of the overall population was 18.2±2.4 months (95% CI: 9.2–27.1). The 1- and 3-year OS rates were 69.8% (95% CI: 0.578–0.818) and 30.1% (95% CI: 0.171–0.431), respectively. The median PFS was 13.9±2.7 months (95% CI: 8.6–19.3). The 1- and 3-year PFS rates were 59.1% (95% CI: 0.461–0.721) and 14.2% (95% CI: 0.132–0.152), respectively. In patients who received full dose dCRT, the median OS and PFS were 25.8±5.4 months (95% CI: 15.2–36.4) and 18.2±4.1 months (95% CI: 10.2–26.2), respectively. In patients who had reduced dose of dCRT or didn’t complete the dCRT course, the median OS and PFS were 12.3±0.3 months (95% CI: 11.7–13.0) and 9.0±2.1 months (95% CI: 4.9–13.2), respectively. There were significant differences in OS and PFS between the two groups (P=0.046 and 0.031, respectively, Figure 2A,B). In patients at stages I-II and III, the median OS and PFS were 39.0±2.3 months (95% CI: 34.5–43.5), 31.8±2.5 months (95% CI: 26.9–36.7) and 16.3±1.5 months (95% CI: 13.4–19.2), 12.3±0.8 months (95% CI: 10.7–13.8), respectively. In patients at stage IVa, the median OS and PFS were 9.1±1.6 months (95% CI: 5.9–12.2) and 6.4±1.2 months (95% CI: 4.0–8.9), respectively. There were also significant differences in OS and PFS according to clinical stages (both P<0.001, Figure 2C,D). In patients aged ≥75 and 70≤ age <75 years, the median OS and PFS were 14.3±4.1 months (95% CI: 6.3–22.3), 11.6±4.9 months (95% CI: 2.0–21.2) and 23.7±5.6 months (95% CI: 12.8–34.6), 15.2±2.2 months (95% CI: 10.9–19.5), respectively. None statistical significance were observed according to age stratification (P=0.140 and 0.138, respectively; Figure 2E,F). In patients achieved CR, the median OS and PFS were 35.1±3.7 months (95% CI: 27.9–42.3) and 23.9±15.8 months (95% CI: 12.6–35.2), respectively. In patients evaluated with Non-CR, the median OS and PFS were 9.5±2.4 months (95% CI: 4.8–14.2) and 6.6±0.9 months (95% CI: 4.7–8.4), respectively, (both P<0.001, Figure 2G,H).
Discussion
In this study, we evaluated the feasibility and efficiency of S-1/CDDP/RT in the setting of dCRT for elderly ESCC patients. Our results showed that the median OS and PFS time were 18.2 and 13.9 months, with the 3-year OS and PFS rates were 30.1% (95% CI: 0.171–0.431) and 14.2% (95% CI: 0.132–0.152), respectively. The survival outcomes seemed comparable with a series of contemporary studies using other treatment regimens for elderly patients. In 2008, Tougeron et al. retrospectively analysed 109 elderly (aged over 70 years) esophageal cancer patients with non-metastatic diseases. Patients received the classical dCRT regimen with CDDP plus 5-Fu or irinotecan, survival results showed that the median OS time was 15.2±2.8 months and disease-free survival was 8.3±7.3 months. Two- and 5-year OS rates were 35.5% and 12.8%, respectively (17). In another study, 33 elderly (aged ≥70 years) patients received dCRT consisted of the continuous infusion of 5-Fu and the intravenous infusion of CDDP combined with 60 Gy radiation therapy. Results showed that the median survival time for the elderly patients was 14.7 months and the 3-year OS rate was 29.3% (18). Compared our survival outcomes with the same treatment regimen for non-elderly esophageal cancer patients, Cho and his co-workers reported the first results of using S-1 and CDDP for unresectable or metastatic ESCC in 2008. Twenty-seven enrolled patients received S-1 and CDDP at doses of 70 mg/m2/day for 14 days and 70 mg/m2 on day 1, respectively, every 3 weeks. Concurrent radiation therapy was delivered with a daily fraction of 2.0 Gy to a total dose of 54 Gy. Results showed that the median OS and PFS times were 16.0±2.4 months (95% CI: 11.2–20.8) and 7.7±2.5 months (95% CI: 2.6–12.74), respectively. In patients at stages II-III, the median OS and PFS were 23.0±5.1 months (95% CI: 13.0–32.9) and 10.6±0.6 months (95% CI: 9.4–11.8), respectively. In patients at stage IV, the median OS and PFS times were 11.6±1.6 months (95% CI: 8.4–14.8) and 5.4±1.6 months (95% CI: 2.2–8.6), respectively (11). In another large sample prospective trial conducted in Japan, 116 locally advanced esophageal cancer patients were assigned to receive S-1/CDDP/RT. The treatment protocol comprised two courses of a 30Gy RT over 3 weeks plus S-1 (80 mg/m2/day) for 2 weeks and a 24-hour CDDP infusion (70 mg/m2) on day 8, and an identical course administered after a 2-week break. Results showed that the median OS time was 2.3 years and the median OS of patients with stage II, III, and IVa disease was 7.0, 2.6, and 1.3 years, respectively. The median PFS time was 1.2 years and the median PFS for patients with stage II, III, and IVa disease was 6.6, 1.6, and 0.6 years, respectively (12). Both of these studies demonstrated a significant difference of survival outcomes according to clinical stages, which was consistent with our findings.
Myelosuppression was the most common treatment-related toxicities for elderly patients in our study and the incidence of moderate-to-severe toxic reactions was apparently lower when compared with dCRT regimen based on CDDP and 5-Fu. In the study which enrolled 33 elderly patients mentioned above, 70.0% (23/33) patients experienced grade ≥3 leucocytopenia. Anemia and thrombocytopenia were also observed in 51.5% and 33.3% patients, respectively. After comparing the clinical outcomes with non-elderly patients, they concluded that it might be difficult to deliver dCRT based on CDDP and 5-Fu in elderly patients because of more substantial toxicities (18). As aforesaid, S-1 is known to be a new generation, orally active fluoropyrimidine, it had been proven to have several advantages over 5-Fu in the setting of dCRT. First, It could prolong the half-life of plasma concentrations of 5-Fu after oral S-1. Second, S-1 could be given on a daily basis, which is effective for irradiation. Recently, Tahara et al. carried out a phase I/II trial of dCRT with S-1 and CDDP to determine the recommended dose of S-1 and evaluate the efficacy and safety of this treatment regimen in patients with stage II-III esophageal cancer. In the phase II part, 38 patients received S-1 and CDDP at doses of 60 mg/m2/d and 75 mg/m2 on day 1, every 3 weeks and concurrent with 50.4 Gy radiation therapy. Toxicity profile revealed that grade 3–4 leucocytopenia and neutropenia was observed in 57.9% and 50% patients, which was comparable with our data for elderly patients (19). Additionally, in the subgroup analysis based on age stratification, our results further showed that patients aged over 75 years had significantly higher incidence of severe leucocytopenia when compared with patients aged between 70 and 75. Although supportive therapy (G-CSF and nutritional support etc.) was performed in the treatment course, less reserve of body function and more comorbidities might be the main reasons for the higher toxicities in this cohort.
It is noteworthy that 38 (67.9%) patients completed dCRT as planned in the present study. Iwase et al. reported that 106 (91.4%) patients completed the treatment course and among these patients, 70 (60.3%) received the planned dose (12). The completion rate was also in line with other studies for elderly esophageal cancer patients. Song et al. reported a retrospective study of 82 elderly esophageal cancer patients receiving dCRT with CDDP and paclitaxel. The completion rate was observed in 67.1% (55/82) patients (20). In another study which investigated the efficiency and safety of using S-1 as single chemotherapy regimen combined with definitive concurrent radiotherapy for elderly patients with esophageal cancer, 51 (75.0%) patients finished dCRT on schedule, including 48 (70.6%) patients without changing treatment regimen (21).
This study is limited by its retrospective design and the relatively small number of patients. Another drawback is the lack of available phase I data determining the dose of S-1 combined with cisplatin-based dCRT for elderly esophageal cancer patients in the literature. Considering all the aspects in the study, large-scale prospective trials for elderly esophageal cancer patients are highly warranted in the near future.
In conclusion, our results showed that the dCRT regimen combined S-1 with CDDP yielded satisfactory survival outcomes but treatment-related toxicities were relatively high, especially for patients aged over 75 years. Physicians should be cautious with adverse events and further study is needed to establish predictive factors that may identify patients at risk for substantial toxicity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of the First Affiliated Hospital of Soochow University (FAHSU No. 2016067). Written informed consent was obtained from all participants.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol 2012;13:790-801. [Crossref] [PubMed]
- Di Pardo BJ, Bronson NW, Diggs BS, et al. The Global Burden of Esophageal Cancer: A Disability-Adjusted Life-Year Approach. World J Surg 2016;40:395-401. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Mohansingh MP. Mortality of oesophagal surgery in the elderly. Br J Surg 1976;63:579-80. [Crossref] [PubMed]
- Poon RT, Law SY, Chu KM, et al. Esophagectomy for carcinoma of the esophagus in the elderly: results of current surgical management. Ann Surg 1998;227:357-64. [Crossref] [PubMed]
- Chino O, Makuuchi H, Machimura T, et al. Treatment of esophageal cancer in patients over 80 years old. Surg Today 1997;27:9-16. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Wakui R, Yamashita H, Okuma K, et al. Esophageal cancer: definitive chemoradiotherapy for elderly patients. Dis Esophagus 2010;23:572-9. [Crossref] [PubMed]
- Fukushima M, Sakamoto K, Sakata M, et al. Gimeracil, a component of S-1, may enhance the antitumor activity of X-ray irradiation in human cancer xenograft models in vivo. Oncol Rep 2010;24:1307-13. [Crossref] [PubMed]
- Cho SH, Shim HJ, Lee SR, et al. Concurrent chemoradiotherapy with S-1 and cisplatin in advanced esophageal cancer. Dis Esophagus 2008;21:697-703. [Crossref] [PubMed]
- Iwase H, Shimada M, Tsuzuki T, et al. Concurrent chemoradiotherapy with a novel fluoropyrimidine, S-1, and cisplatin for locally advanced esophageal cancer: long-term results of a phase II trial. Oncology 2013;84:342-9. [Crossref] [PubMed]
- Li YH, Qiu MZ, Xu JM, et al. S-1 plus cisplatin versus fluorouracil plus cisplatin in advanced gastric or gastro-esophageal junction adenocarcinoma patients: a pilot study. Oncotarget 2015;6:35107-15. [PubMed]
- Thomson RM, Armstrong JG, Looke DF. Gastroesophageal reflux disease, acid suppression, and Mycobacterium avium complex pulmonary disease. Chest 2007;131:1166-72. [Crossref] [PubMed]
- Ji Y, Qiu G, Sheng L, et al. A phase I dose escalation study of S-1 with concurrent radiotherapy in elderly patients with esophageal cancer. J Thorac Dis 2016;8:451-8. [Crossref] [PubMed]
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341-6. [Crossref] [PubMed]
- Tougeron D, Di Fiore F, Thureau S, et al. Safety and outcome of definitive chemoradiotherapy in elderly patients with oesophageal cancer. Br J Cancer 2008;99:1586-92. [Crossref] [PubMed]
- Takeuchi S, Ohtsu A, Doi T, et al. A retrospective study of definitive chemoradiotherapy for elderly patients with esophageal cancer. Am J Clin Oncol 2007;30:607-11. [Crossref] [PubMed]
- Tahara M, Fuse N, Mizusawa J, et al. Phase I/II trial of chemoradiotherapy with concurrent S-1 and cisplatin for clinical stage II/III esophageal carcinoma (JCOG 0604). Cancer Sci 2015;106:1414-20. [Crossref] [PubMed]
- Song T, Zhang X, Fang M, et al. Concurrent chemoradiotherapy using paclitaxel plus cisplatin in the treatment of elderly patients with esophageal cancer. Onco Targets Ther 2015;8:3087-94. [PubMed]
- Lv S, Fang M, Yang J, et al. Long-term results of definitive concurrent chemoradiotherapy using S-1 in the treatment of geriatric patients with esophageal cancer. Onco Targets Ther 2016;9:5389-97. [Crossref] [PubMed]


