Treatment of breast cancer in young women: do we need more aggressive therapies?
Introduction
Breast cancer in young patients is an important topic for manifold reasons. First of all, the prevalence of breast cancer in pre-menopausal women has been steadily increasing in several countries over the last years (1,2). Moreover, the management of breast cancer in young patients (<35 or <40 years) solicits an integrated approach taking into account relevant issues such as fertility preservation and pregnancy, apart from a long-life expectancy.
Overall, young patients have been reported to be associated with an increased risk of recurrence and death, as well as with unfavorable clinical and biological characteristics when compared to older patients (3-7).
Although it’s clear that breast cancer in young women presents more frequently an aggressive phenotype with a consequently adverse outcome, controversies exist regarding the optimal treatment in this population and if more aggressive therapies are really crucial.
Furthermore very young women with this disease are faced with personal, family, professional, and quality-of-life issues that further complicate the phase of treatment decision-making.
Focus on adjuvant chemotherapy of breast cancer in young women
Age is not clearly associated to a specific response to chemotherapy. There are in fact some controversial data about the potential role of age as predictive factor.
The meta-analysis performed by Early Breast Cancer Trialists’ Collaborative Group showed that polychemotherapy in women less than 50 years was associated with a recurrence rate of 41% compared to 53% of control group with a 15-year gain of 12%, while the 15-year gain was 4% for women aged more than 50 years. The effect of chemotherapy on recurrence rate and mortality was independent of age. The data have been subdivided into 10-year bands of age at entry; the mean annual reduction of risk of relapse attributable to chemotherapy (mainly CMF and anthracyclines) was 40% in patients less than 40, 36% in patients 40-49 and 23% in patients 50-59 (8).
In women younger than 50 years and oestrogen receptor positive (ER+) tumors, adjuvant polychemotherapy is associated with an annual reduction in mortality of 31% [standard error (SE) =0.10]. In this subgroup of patients, tamoxifen is also very effective with an annual reduction in mortality ranging from 39% (SE=0.12) in women younger than 40 years to 24% in women aged 40-49 years (8).
However, when ER status is taken into account, age disappears as an independent prognostic factor for the benefit of chemotherapy with all ER-negative patients benefiting from chemotherapy at the same extent (9).
Data with more recent regimens including taxanes are much more controversial, with some studies suggesting a higher and others a lower benefit in younger women. Obviously, these data have to be carefully interpreted, the effects observed being in part related to the degree of amenorrhea induced by the diverse regimens (10).
The most recent meta-analysis of EBCTCG compared different polychemotherapy regimens, including also the taxanes. In all meta-analyses involving taxane-based or anthracycline-based regimens, proportional risk reductions were little affected by age. Hence, largely independent of age (up to at least 70 years) or the tumour characteristics currently available to us for the patients selected to be in these trials, some taxane-plus-anthracycline-based or higher-cumulative-dosage anthracycline-based regimens (not requiring stem cells) reduced breast cancer mortality by, on average, about one-third (11).
Chemotherapy in ER-positive breast cancer in young women: endocrine effect of chemotherapy
Available adjuvant treatments for premenopausal endocrine-responsive breast cancer patients include chemotherapy and/or tamoxifen and luteinizing hormone-releasing hormone (LH-RH) agonists.
Chemotherapy exerts some of its effect via an endocrine mechanism in premenopausal women with ER-positive tumors (12).
It is important to focus on endocrine effects (suppression of endocrine ovarian function) of chemotherapy in premenopausal women. The endocrine effects of chemotherapy vary with age. Goodwin et al. examined factors predicting onset of menopause in a cohort of premenopausal women with newly diagnosed breast cancer receiving either adjuvant CMF, cyclophosphamide, epirubicin, and fluorouracil (CEF), tamoxifen, or no treatment. They demonstrated that two factors, age and use of systemic chemotherapy, are important predictors of menopause onset in premenopausal women with newly diagnosed breast cancer, with the risk that began to increase at age 35 (13).
Moreover it is known that the incidence of amenorrhea is proportional to the duration of chemotherapy (14).
Data in the literature support a role for ovarian function suppression in the adjuvant program of pre-menopausal patients.
Between 1978 and 1993 the International Breast Cancer Study Group (IBCSG) treated 3,700 premenopausal and perimenopausal patients with various timing and duration of adjuvant cyclophosphamide, methotrexate, and fluorouracil (CMF with or without low-dose prednisone and oophorectomy) in Trials I, II, V and VI. 314 of these women were less than 35 years old at randomisation. In these trials patients were not routinely offered hormonal therapy following chemotherapy. Trial I investigated the addition of low-dose prednisone to a cyclophosphamide-methotrexate-fluorouracil (CMF) combination in patients with one to three positive axillary nodes. In trial II, patients with four or more positive axillary nodes were randomised to 1 year of CMF and low-dose prednisone (CMFP) or to a surgical oophorectomy and CMFP. In Trial V and VI patients received only chemotherapy but no any kind of hormonal therapy. The failure to achieve chemotherapy-induced amenorrhea was associated with an increased risk of relapse among pre-menopausal patients with ER-positive tumors [hazard ratios (HR), 1.67; 95% confidence interval (CI), 1.19-2.34; P=0.003] in this retrospective analysis of IBCSG Trials I, II, V and VI. Moreover in these trials, younger patients with ER-positive tumors had a significantly worse prognosis than did younger patients with ER-negative tumors (10-year DFS was 25% for ER-positive tumors versus 47% for ER-negative tumors; P=0.014). In contrast, among older patients, the prognosis was similar for patients with ER-positive tumors compared to patients with ER-negative tumors (10-year DFS was 45% versus 46%; P=0.27). The interaction between age and ER status on outcome was statistically significant (P=0.002) (15).
A retrospective cohort study of a National Cancer Institute of Canada Clinical Trials Group indicated that the achievement of amenorrhea at 12 months was significantly associated with relapse-free survival and overall survival (16).
Finally, the results of IBCSG trail 13-93 showed that premenopausal patients with ER-positive tumors who achieved chemotherapy-induced amenorrhea had a significantly improved outcome (HR for amenorrhea v no amenorrhea =0.61; 95% CI, 0.44 to 0.86; P=0.004), whether or not they received tamoxifen (17).
A pooled analysis of patients 40 years old or younger enrolled in different EORTC trials demonstrated that hormone receptor-positive patients experienced no survival advantage of prolonged adjuvant CMF chemotherapy compared with hormone receptor-negative patients. However, in patients who did not receive adjuvant chemotherapy, hormone receptor-positive status was associated with improved survival rates compared with hormone receptor-negative status. In overall multivariate analyses, both ER-positive status and PgR-positive status remained independent prognostic factors of OS. Young patients with hormone receptor-positive tumors benefit less from adjuvant systemic chemotherapy than patients with hormone receptor-negative tumors. These results confirm that chemotherapy alone cannot be considered optimal adjuvant systemic treatment in breast cancer patients 40 years old or younger with hormone receptor-positive tumors (18).
These analyses of treatment outcome leads to the hypothesis that the endocrine effects of chemotherapy alone were insufficient for patients in the younger age group with endocrine-responsive tumors, for whom suppression of estradiol production might be essential.
However, recent epidemiological data seem to show and confirm the recent attitude to use chemotherapy but also better/optimal endocrine treatment (i.e., LHRH-analogue plus Tamoxifen) for young patients with endocrine-responsive disease (19).
A recent SEER population-base study in fact showed that HR for mortality in women 40-50 with ER positive BC but also in women <40 with ER positive had improvements over time.
In ER negative patients, the degree of improvements over time was less than that seen in ER positive women. Authors conclude that therefore, mortality improvements in young women with ER positive BC may be attributed to treatment advances with endocrine agents (19).
However the question of whether additional benefit can be obtained from ovarian suppression in premenopausal patients receiving tamoxifen is now being directly addressed by the global Suppression of Ovarian Function Trial (SOFT) coordinated by the IBCSG on behalf of the Breast International Group and the North American Breast Cancer Intergroup. SOFT compares tamoxifen alone versus ovarian function suppression plus tamoxifen versus ovarian function suppression plus exemestane for patients with steroid hormone receptor-positive tumors who remain premenopausal after adjuvant chemotherapy or for whom tamoxifen alone is considered reasonable treatment option.
Chemotherapy in ER-negative breast cancer in young women
Regardless of the age of premenopausal patients with ER-negative tumors, adjuvant chemotherapy appears to be a very important component of a successful treatment regimen.
Evaluation of data from NSABP, IBCSG and SWOG trails, showed that the difference in outcome with respect to age group (young versus old patients) is much smaller for patients with ER-negative tumors compared to patients with ER-positive tumors. No difference was found about relative risk of relapse comparing patients less than 35 years old with those 35 years of age and older with ER-negative disease who received adjuvant chemotherapy. Therefore the beneficial effects of chemotherapy might be similar for younger and older premenopausal women for the ER-negative cohort (20).
In the NSABP Trial B-13, specifically designed for ER-negative disease, the effect of chemotherapy compared with no adjuvant treatment in women less than 50 years old is overwhelming, corresponding to a 38% reduction in the risk of relapse. In this setting of ER-negative disease, the magnitude of the estimated effect of chemotherapy is the same for younger as for older patients although, because of the smaller sample size, the result for the younger group is statistically uncertain (21).
Colleoni et al. evaluated biological features, treatment recommendations and prognosis for 841 premenopausal patients with pT1-3, pN0 and M0, operated at European Institute of Oncology, Milan, Italy from 1997 to 2001. Treatment modalities were well balanced between the young and older patients in the subgroup of endocrine unresponsive disease; in this subgroup, a statistically significant difference in DFS but not OS was observed for very young patients (below 35 years) versus older patients in univariate analysis (HR=3.26, 95% CI, 1.14 to 9.33, P<0.0196 for DFS; HR=2.12, 95% CI, 0.60 to 7.51; P=0.24 for OS). The association disappeared in multivariate analysis (17).
Recent data of GeparTrio neoadjuvant study suggest that young age is constantly associated with greater benefit from preoperative anthracycline-taxane-based chemotherapy. In this trial about 17.4% of the patients were below the age of 40 years, pCR was significantly higher in patients under the age of 40 years compared to those 40 years or older. The highest pCR rate could be detected for those under 40 years with an ER/PgR negative (P=0.001) tumor. When a tumor was triple negative pCR rates were as high as 57% in the <40 years population compared to 34% in the patients ≥40 years (P<0.0001). In the triple negative setting, age was the only independent predictive factor for chemotherapy response in this setting (22).
Another neoadjuvant trial evaluated cisplatin in twenty-eight patients with triple-negative breast cancer to identify specific biomarkers predictors of response.
The study showed a strong association between younger age and good response (P=0.001 based on quartiles of age, according to Miller-Payne score; significant even after Bonferroni adjustment for multiple comparisons; when the two BRCA1 mutations carriers were excluded, P=0.001).
However age was not significantly associated with pCR (P=0.13) or clinical response (P=0.46) (23).
A more aggressive therapy: two examples
Dose-dense chemotherapy
Two systematic reviews and meta-analyses of the existing data from randomized controlled trials regarding the efficacy and toxicity of the dose-dense adjuvant chemotherapy were published.
The first meta-analysis showed that patients who received dose-dense chemotherapy had better overall survival [HR of death =0.84, 95% CI =0.72 to 0.98, P=0.03] and better disease-free survival (HR of recurrence or death =0.83, 95% CI, 0.73 to 0.94, P=0.005) than those on the conventional schedule; no benefit was observed in patients with hormone receptor-positive tumors (24).
The second meta-analysis demonstrated that dose-dense therapy can improve DFS (3,356 patients; HR=0.83; 95% CI, 0.73e0.95; P=0.005), independent of hormone receptor expression status; there was no OS benefit with dose-dense therapy (25).
However both meta-analyses didn’t perform efficacy analyses according to the age of patients.
In the study of Venturini et al. 1,214 patients with early-stage breast cancer were randomly assigned to receive six cycles of FEC 14 (administered every 14 days) or of FEC 21 (administered every 21 days). At a median follow-up of 10.4 years, no statistically significant difference in the hazard of death [hazard ratio (HR) =0.87, 95% CI, 0.67 to 1.13] or recurrence (HR=0.88, 95% CI, 0.71 to 1.08) was found between FEC 14 and FEC 21 groups after adjustment by multivariable analysis. Although the study was underpowered for subset analysis, authors observed a suggestion of higher efficacy associated with the FEC 14 regimen than with the FEC 21 regimen among patients younger than 50 years; these patients had a statistically significant 34% reduced risk of recurrence (HR=0.66, 95% CI, 0.46 to 0.94) and a non-statistically significant 27% reduced risk of death (HR=0.73, 95% CI, 0.46 to 1.16). This greater efficacy seems not to be mediated by a greater activity of FEC 14 in suppressing ovarian function, because the rate of chemotherapy-induced amenorrhea was virtually identical in the two arms (26).
The INT 9742 trial randomized about 2,000 patients to receive sequential or concurrent chemotherapy with anthracyclines and taxanes every two or three weeks. Dose-dense treatment improved the primary end point, DFS [risk ratio (RR) =0.74; P=0.010], and OS (RR=0.69; P=0.013). Multivariate analysis didn’t show a different risk of death among pre-menopausal and post-menopausal patients (27).
Finally, no studies specifically evaluated or analyzed the impact of the dose-dense chemotherapy in very young patients (below 35 or 40 years), apart from a controversial exploratory data about premenopausal status; therefore, even if the dose-dense regimens are apparently feasible about acute and late toxicities, they cannot be considered a standard approach in very young patients with early breast cancer.
Dose-intensive /high-dose chemotherapy
In 1995, the International Breast Cancer Study Group (IBCSG) initiated a clinical trial (Trial 15-95) to examine the role of dose-intensive epirubicin and cyclophosphamide (DI-EC) versus conventional adjuvant chemotherapy for patients with high-risk early breast cancer. After prolonged follow-up, DI-EC significantly improved DFS, but the effect was observed only in patients with ER-positive disease, leading to the hypothesis that efficacy of DI-EC may relate to its endocrine effects.
A STEPP analysis, conducted in order to ascertain the magnitude of the effect of DI-EC in patients with ER-positive tumors according to age, showed a visual trend suggesting a larger effect for DI-EC in younger patients, therefore supporting a possible correlation between the achievement of ovarian function suppression and efficacy of DI-EC., even if the interaction of age and treatment was not statistically significant (P=0.54) (28).
Other studies exploring the activity of high-dose chemotherapy described a more pronounced effect of high dose chemotherapy in younger patients.
A trend towards an advantage for younger women (age <35 years) and women with four to nine involved axillary lymph nodes was also shown in the Italian study of the Michelangelo Group, in spite of a lack of an overall benefit after median 5 years of follow-up (29).
In general, adjuvant high-dose chemotherapy (HDC) with autologous hematopoietic stem-cell transplantation (AHST) for high-risk primary breast cancer has not been shown to prolong survival. Moreover individual trials have had limited power to show overall benefit or benefits within subsets.
However some retrospective subgroup analyses showed benefit from high-dose chemotherapy independent of hormone receptor status and age. Effects were more pronounced in young patients, but no data are available on the effect according to age, amenorrhea in endocrine-responsive disease, and in those with hormone receptor-negative disease (29,30).
A recent meta-analysis of individual patient data from 15 randomized adjuvant breast cancer trials including 6,210 patients showed that after a median follow-up of 6 years high-dose chemotherapy prolong relapse-free survival [hazard ratio (HR), 0.87; 95% CI, 0.81 to 0.93; P=0.001] but not overall survival (OS; HR, 0.94; 95% CI, 0.87 to 1.02; P=0.13). Younger patients had a significantly better RFS on HDC than did older patients. However for overall survival, no covariates had statistically significant interactions with treatment effect, and no subsets evinced a significant effect of high-dose chemotherapy (31).
The sum of these results limit the use of high-dose chemotherapy in breast cancer.
The advantage in some subsets of patients was restricted to some retrospective analyses with low study power.
In clinical decision making, any benefit in recurrence or survival must be weighted against the greater toxicities of HDC.
Individual studies have reported that the quality of life among patients receiving HDC is lower during treatment than that among the patients receiving control (32).
These findings appear more relevant in young patients in whom we have to consider the impact of acute but also late toxicities in relation to long life-expectancy, too. Reliable evidence of benefit is required to justify the burden and expense of dose-intensive therapy and the results in patients with ER-positive disease raise the hypothesis that efficacy of DI-EC may relate to its endocrine effects. There are surely less costly ways of offering endocrine therapy to very young patients with endocrine-responsive breast cancer.
Biology of breast cancer in young women
Recent studies have examined the distribution of breast cancer immunohistochemical and molecular subtypes and gene expression signatures to evaluate if breast cancer in young women is enriched with aggressive subtypes and also to question whether breast cancer diagnosed at a young age has a unique biology. The findings of these field researches could be relevant to discriminate prognostic subgroups in young patients, but also to understand if young age alone can be an indicator for adjuvant chemotherapy.
Breast cancer is a heterogeneous disease and gene expression studies have identified molecularly distinct subtypes with prognostic implications across multiple treatment settings. The immunohistochemical evaluation of ER, progesterone receptor (PgR), Ki-67 and HER2 may be considered a surrogate means for identifying the molecular subtypes of breast cancer.
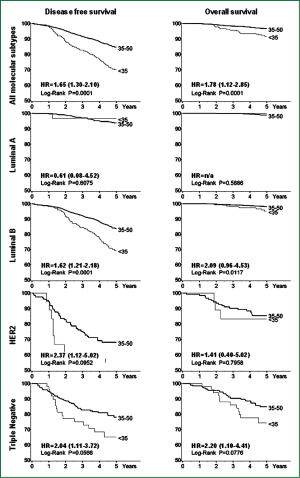
Cancello et al. investigated the prognosis of very young patients (below 35 years) compared to older premenopausal patients (aged 35-50) using an immunohistochemical classification. The analysis was based on data from 2,970 patients, of whom 315 were aged less than 35 years. According to the immunohistochemical classification, in the group of patients aged <35 years, there were less tumors identified as Luminal A (9.2% versus 21.2%) and more Triple Negative tumors (16.2% versus 7.5%; P<0.0001) than in older patients, apart from a higher prevalence of high grade tumors and a higher percentage of tumors with peri-vascular invasion.
More importantly, in the same study, patients <35 years of age presented a significantly increased risk of recurrence and death [hazards ratio HR =1.65, 95% CI 1.30-2.10 and HR=1.78, 95% CI, 1.12-2.85, respectively] when compared with older patients with similar characteristics of disease. Very young patients with tumors classified as Luminal B, HER2 and Triple Negative were at increased risk of poorer DFS (HR=1.62, 95% CI, 1.21-2.18; HR=2.37, 95% CI, 1.12-5.02 and HR=2.04, 95% CI, 1.11-3.72, respectively), while in the Luminal B and Triple Negative subtypes, patients <35 years had a twofold higher risk of death compared with older patients (Figure 1). In this series very young patients with triple negative and HER2-subtype breast cancer received the same percentage of chemotherapy compared with older patients, while patients aged less than 35 years with Luminal B tumors receive more chemotherapy and more LHRH-analogue + tamoxifen combination therapy than older patients with the same subtype disease (33).
In 2008 a large-scale genomic analysis was published. In this study two age-specific cohorts (young: ≤45 years, n=200; older: ≥65 years, n=211) were compared by prognosis, clinicopathologic variables, mRNA expression values, single gene analysis, and gene set enrichment analysis (GSEA). Tumors arising in young women had significantly lower ERα mRNA (P≤0.0001), ERα (P=0.02), and progesterone receptor (PR) expression (P<0.0001), but higher HER-2 (P<0.0001) and epidermal growth factor receptor (EGFR) expression (P<0.0001). Exploratory analysis (GSEA) revealed 367 biologically relevant gene sets significantly distinguishing breast tumors arising in young women. Combining clinicopathologic and genomic variables tumors arising in young women demonstrated that younger age and lower ERβ and higher EGFR mRNA expression were significant predictors of inferior DFS (34).
However after some years same authors chose to reanalyze their previous data set to evaluate the relationship between age and breast cancer subtype, and to account for potential confounding variables not previously included. First of all, they found that there was a significant association between subtype and age (P=3.8e-06). Specifically, a higher proportion of younger women were diagnosed with basal-like [odds ratio (OR), 12.27; 95% CI, 3.96 to 45.0] and HER2-enriched (OR, 4.63; 95% CI, 1.50 to 16.48) breast tumors. More interesting, the correction for the significant clinicopathologic features (grade, subtype, sample source) with the adjusted model yielded zero gene differences (q<0.05) between breast tumors of previously defined age groups in two different data sets (35).
More recently, a comprehensive analysis was conducted to clarify the relevance of several published prognostic gene signatures in young women (≤40) and to determine whether young age is truly associated with unique disease biology.
In about 2,901 patients, authors observed a significantly higher risk of relapse in patients of 40 years or less than in older age groups (P
More interestingly, authors identified a total of 41 genes and 13 gene sets as potential candidate age-related genes and pathways aberrations reported in previous literature data. Within a cohort of untreated patients the expression of 16 genes and gene sets were found to be significantly age dependent after adjustment. In the cohort of treated patients authors found that 12 out of the 16 were still significantly associated with age after adjustment. The common themes associated with young age were enrichment of biological processes related to immature mammary cell populations (RANKL, c-kit, BRCA1-mutated phenotype, mammary stem cells, and luminal progenitors cells), and growth factor signaling [mitogen—activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)-related]. There was also downregulation of apoptosis-related genes (36).
The few studies published on intrinsic biology of breast cancer in young women showed as only conclusive result that breast cancer in young women present more frequently an aggressive phenotype.
The analysis of immuno-defined subtypes seems to show that the behaviour of a specific subtype in a young patient is intrinsically more aggressive than in an older patient (33).
However the gene-signature analyses showed contradictory results, on the one hand showing that age alone does not appear to provide an additional layer of biologic complexity above that of breast cancer subtype and grade, on the other hand suggesting that breast cancer arising at a young age is biologically distinct beyond subtype distribution and is enriched with unique molecular processes (35,36).
Final considerations and conclusions
The available published data don’t suggest a specific medical treatment approach for the very young patients with breast cancer.
The indications for and the choice of type of adjuvant systemic treatment for invasive breast cancer should be driven, as in other age categories, by the biological characteristics of the tumours, as the immunohistochemical-defined subtypes, the tumour stage and patient’s comorbidities and preferences. Furthermore the type of systemic treatment of early breast cancer is independent of BRCA or any other constitutional genetic status.
Therefore, for the time being, young age alone should not be a reason to prescribe more aggressive therapies and there are no evidence to recommend a specific chemotherapy regimen for young women.
The association with a more aggressive biology should be better understand so to aid management of young patients with breast cancer, and more important, to tailor treatment investigations so to clarify if we need new modalities of treatment or, simply we have to use better the modalities available today.
Additionally, a better understanding of the oncogenic signaling pathways of breast cancer arising in young women so to elucidate if breast cancer in youth is a unique biologic entity could enable us to better tailor treatments that could be offered to young women.
Prospective data from the randomized trials probably will help to re-assess the prognosis and benefit of chemotherapy according to age and tumour biology in the modern era.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Bouchardy C, Fioretta G, Verkooijen HM, et al. Recent increase of breast cancer incidence among women under the age of forty. Br J Cancer 2007;96:1743-6. [PubMed]
- Brinton LA, Sherman ME, Carreon JD, et al. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst 2008;100:1643-8. [PubMed]
- Walker RA, Lees E, Webb MB, et al. Breast carcinomas occurring in young women (<35 years) are different. Br J Cancer 1996;74:1796-800. [PubMed]
- Chung M, Chang HR, Bland KI, et al. Younger women with breast carcinoma have a poorer prognosis than older women. Cancer 1996;77:97-103. [PubMed]
- Winchester DP, Osteen RT, Menck HR. The National Cancer Data Base report on breast carcinoma characteristics and outcome in relation to age. Cancer 1996;78:1838-43. [PubMed]
- Albain KS, Allred DC, Clark GM. Breast cancer outcome and predictors of outcome: are there age differentials? J Natl Cancer Inst Monogr 1994;(16):35-42. [PubMed]
- Colleoni M, Rotmensz N, Robertson C, et al. Very young women (<35 years) with operable breast cancer: features of disease at presentation. Ann Oncol 2002;13:273-9. [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Clarke M, Coates AS, et al. Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level meta-analysis of randomised trials. Lancet 2008;371:29-40. [PubMed]
- Swain SM, Jeong JH, Geyer CE Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med 2010;362:2053-65. [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432-44. [PubMed]
- Pagani O, O’Neill A, Castiglione M, et al. Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: results of the International Breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer 1998;34:632-40. [PubMed]
- Goodwin PJ, Ennis M, Pritchard KI, et al. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol 1999;17:2365-70. [PubMed]
- Goldhirsch A, Gelber RD, Castiglione M. The magnitude of endocrine effects of adjuvant chemotherapy for premenopausal breast cancer patients. The International Breast Cancer Study Group. Ann Oncol 1990;1:183-8. [PubMed]
- Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet 2000;355:1869-74. [PubMed]
- Parulekar WR, Day AG, Ottaway JA, et al. Incidence and prognostic impact of amenorrhea during adjuvant therapy in high-risk premenopausal breast cancer: analysis of a National Cancer Institute of Canada Clinical Trials Group Study--NCIC CTG MA.5. J Clin Oncol 2005;23:6002-8. [PubMed]
- International Breast Cancer Study Group, Colleoni M, Gelber S, et al. Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: International Breast Cancer Study Group Trial 13-93. J Clin Oncol 2006;24:1332-41. [PubMed]
- van der Hage JA, Mieog JS, van de Vijver MJ, et al. Efficacy of adjuvant chemotherapy according to hormone receptor status in young patients with breast cancer: a pooled analysis. Breast Cancer Res 2007;9:R70. [PubMed]
- Ademuyiwa FO, Groman A, Hong CC, et al. Time-trends in survival in young women with breast cancer in a SEER population-based study. Breast Cancer Res Treat 2013;138:241-8. [PubMed]
- Goldhirsch A, Gelber RD, Yothers G, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr 2001;(30):44-51. [PubMed]
- Fisher B, Redmond C, Wickerham DL, et al. Systemic therapy in patients with node-negative breast cancer. A commentary based on two National Surgical Adjuvant Breast and Bowel Project (NSABP) clinical trials. Ann Intern Med 1989;111:703-12. [PubMed]
- Huober J, von Minckwitz G, Denkert C, et al. Effect of neoadjuvant anthracycline-taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat 2010;124:133-40. [PubMed]
- Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 2010;28:1145-53. [PubMed]
- Bonilla L, Ben-Aharon I, Vidal L, et al. Dose-dense chemotherapy in nonmetastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. J Natl Cancer Inst 2010;102:1845-54. [PubMed]
- Lemos Duarte I, da Silveira Nogueira Lima JP, Passos Lima CS, et al. Dose-dense chemotherapy versus conventional chemotherapy for early breast cancer: a systematic review with meta-analysis. Breast 2012;21:343-9. [PubMed]
- Venturini M, Del Mastro L, Aitini E, et al. Dose-dense adjuvant chemotherapy in early breast cancer patients: results from a randomized trial. J Natl Cancer Inst 2005;97:1724-33. [PubMed]
- Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003;21:1431-9. [PubMed]
- Colleoni M, Sun Z, Martinelli G, et al. The effect of endocrine responsiveness on high-risk breast cancer treated with dose-intensive chemotherapy: results of International Breast Cancer Study Group Trial 15-95 after prolonged follow-up. Ann Oncol 2009;20:1344-51. [PubMed]
- Gianni A, Bonadonna G. Five-year results of the randomized clinical trial comparing standard versus high-dose myeloablative chemotherapy in the adjuvant treatment of breast cancer with >3 positive nodes. Proc Am Soc Clin Oncol 2001;20:abstr 80.
- Rodenhuis S, Bontenbal M, van Hoesel QG, et al. Efficacy of high-dose alkylating chemotherapy in HER2/neu-negative breast cancer. Ann Oncol 2006;17:588-96. [PubMed]
- Berry DA, Ueno NT, Johnson MM, et al. High-dose chemotherapy with autologous hematopoietic stem-cell transplantation in metastatic breast cancer: overview of six randomized trials. J Clin Oncol 2011;29:3224-31. [PubMed]
- Farquhar CM, Marjoribanks J, Lethaby A, et al. High dose chemotherapy for poor prognosis breast cancer: systematic review and meta-analysis. Cancer Treat Rev 2007;33:325-37. [PubMed]
- Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (PubMed]
- Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 2008;26:3324-30. [PubMed]
- Anders CK, Fan C, Parker JS, et al. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol 2011;29:e18-20. [PubMed]
- Azim HA Jr, Michiels S, Bedard PL, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res 2012;18:1341-51. [PubMed]


