Pulmonary vein stump thrombosis after left pneumonectomy, diagnosed based on a high plasma D-dimer level: a case report
Introduction
Pulmonary vein stump thrombosis (PVT) was reported first in 1989 by Seki et al. (1). A total of 23 cases have been reported so far, and some have had severe complication due to PVT. Two retrospective studies were conducted and they pointed out that PVT is seen most commonly after left upper lobectomy (LUL) and may not be preventable because of the technical difficulty in shortening the stump of the left upper pulmonary vein (PV) (2,3). Furthermore, a PVT diagnosis was made by follow-up contrast CT or only after a severe thrombotic event occurred. We experienced a case in which PVT was discovered before it induced a severe complication by measuring the D-dimer levels after left pneumonectomy. D-dimer levels can be diagnostic for PVT in the early postoperative state of surgical resection of the left lung.
Case presentation
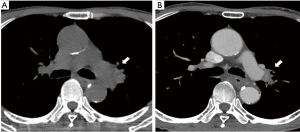
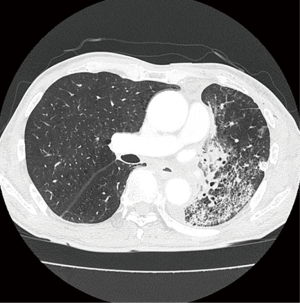
A 69-year-old male smoker was referred to our hospital for the examination of a left pulmonary hilar mass found on computed tomography (CT) at a previous hospital. The mass was revealed to be a squamous cell carcinoma measuring 7 cm in size (Figure 1A) with subcarinal lymph node metastases (cT3N2M0, cStage IIIA). Neoadjuvant chemoradiotherapy (S-1: orally at 40 mg/m2 twice a day on days 1 to 14 and 22 to 36 + Cisplatin: 60 mg/m2 on days 1 and 22, radiation: 40 Gray/20 fractions beginning on day 1) was performed as described previously (4), and the mass was reduced in size by 33%, which was considered a partial response under the RESIST criteria (Figure 1B). We then performed left upper sleeve lobectomy under the patient’s very carefully informed consent. The plasma D-dimer level before the operation was <0.5 µg/mL. During the operation, we performed LUL with plasty of the bronchus and pulmonary artery under proper systemic heparinization by administering 3,000 U of heparin. The X-ray radiolucency of the residual left lung decreased daily after the operation, which forced us to perform contrast-enhanced CT (CECT) on postoperative day (POD) 5. The CECT images revealed ground-grass opacity and the thickening of the interlobular septum in the residual left lower lobe (Figure 2). Any thrombus in the PV was not detected, but the obstruction of pulmonary artery was also pointed out. Based on these findings, the patient was diagnosed with pulmonary congestion of the residual left lobe, and underwent emergent left completion pneumonectomy. We found the kinked pulmonary artery during the operation, which was thought to be the reason for the obstruction.
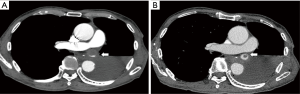
The postoperative course of the second operation was uneventful and the patient was about to be discharged on POD 12 when the laboratory data showed increased serum creatinine and plasma D-dimer levels (1.34 mg/dL, and 17.4 µg/mL respectively). Due to concerns of renal thrombosis, a CECT scan was taken immediately. A giant thrombus was found at the stump of the left superior pulmonary vein (LSPV) (Figure 3A), which prompted us to start anticoagulant therapy with apixaban (10 mg/day). Renal thrombosis was not evident on the CECT scan. The serum creatinine and plasma D-dimer levels decreased soon after the initiation of anticoagulant therapy (0.83 mg/dL, and 3.1 µg/mL, respectively, on POD 30), and CECT conducted on POD 30 (18 days after the anticoagulant therapy) showed remarkable reduction in the thrombus size (Figure 3B).
The patient was discharged on POD 30 without any further complications.
Discussion
PVT is regarded as a rare complication after lobectomy, but in two retrospective studies involving 344 patients altogether, the frequency of PVT was reported to be as high as 15.0% among patients after LUL (3.5% if all patients were included) (2,3). PVT is most commonly seen after LUL, and is thought to be due to the long LSPV stump which creates a turbulent flow inside that leads to thrombus formation. It has also been pointed out that shortening the LSPV stump is sometimes impossible due to technical issues, making it difficult to prevent PVT (3). There have been 23 cases reported thus far, and all of them are listed in Table 1. Of note, 11 of the 23 cases (48%) suffered from thromboembolic events of varying severity that led to the PVT diagnosis, while the other 12 cases were discovered incidentally on follow-up CECT scan. Only 1 case showed slightly increased plasma D-dimer levels (1.7 µg/mL) at PVT presentation, however the absolute value was not diagnostic, and the key finding in that case was also a CECT scan (12). The present case is the first in which a high plasma D-dimer level led to the PVT diagnosis.
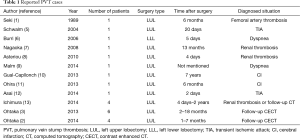
Full table
D-dimer is a stable end-product of fibrin degradation by plasmin and its levels represent the extent of fibrin formation and degradation; therefore, it is considered an excellent marker of a hypercoagulative state, such as that seen with disseminated intravascular coagulation (DIC) syndrome (14). D-dimer levels also increase after surgical events even in the absence of any complications, but their value is much higher when deep vein thrombosis (DVT) is complicated (14). If PVT results from hemostasis in the LSPV stump as explained above, its etiology is similar to that of DVT; we assume that there should be some differences in the extent of the increase in the D-dimer levels after LUL once PVT has occurred. It was reported that D-dimer levels started to rise significantly at POD 3–4, peaking at 4.0 µg/mL after general abdominal surgery and at 14.7 µg/mL after orthopedic surgery at POD 5–7 before gradually decreasing to normal levels at around POD 30 (both cases were without major complications) (15,16). In the present case, the peak was unclear but the value of 17.4 µg/mL at POD 12 was high enough to prompt further investigations. In addition, the D-dimer values before the first and the second operation were <0.5 and 2.5 µg/mL respectively.
In the reported 23 cases, PVT diagnoses were made through CECT scans that were conducted several months after LUL or right after embolic events had occurred. One possible reason that D-dimer levels were not significantly increased in these cases may have been that the timing of their measurement was too early or too late after the onset of PVT. An ongoing multicenter observational study to investigate the incidence of pulmonary venous thrombosis and of cerebral infarction after radical surgery for lung cancer may clarify in part the significance of D-dimer in diagnosing PVT and the appropriate timing for testing D-dimer expression (University Hospital Medical Information Network ID: 000017528).
In conclusion, the present case suggested that the measurement of D-dimer levels may be useful as a screening test for PVT after LUL or left pneumonectomy. PVT may be a much more common complication after LUL than previously assumed; however, an effective method of preventing it has yet to be established. Thromboembolic events associated with PVT can be life-threatening, and a retrospective study found that the rate of cerebral infarction was extremely high, especially after LUL (3,17). Thromboembolic events have deleterious effects on the outcome after surgery (18). The early detection of PVT by measuring D-dimer levels after surgical resection of the left lung may help improve the prognosis, but further study is needed to determine the clinical significance of PVT and D-dimer testing after lung surgeries.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Seki M, Endo M, Kidani M, et al. A rare case of left atrial thrombus after left upper pulmonary lobectomy. Nihon Kyobu Geka Gakkai Zasshi 1989;37:1371-5. [PubMed]
- Ohtaka K, Hida Y, Kaga K, et al. Left upper lobectomy can be a risk factor for thrombosis in the pulmonary vein stump. J Cardiothorac Surg 2014;9:5. [Crossref] [PubMed]
- Ohtaka K, Hida Y, Kaga K, et al. Thrombosis in the pulmonary vein stump after left upper lobectomy as a possible cause of cerebral infarction. Ann Thorac Surg 2013;95:1924-8. [Crossref] [PubMed]
- Yamaguchi M, Toyokawa G, Ohba T, et al. Preoperative concurrent chemoradiotherapy of S-1/cisplatin for stage III non-small cell lung cancer. Ann Thorac Surg 2013;96:1783-9. [Crossref] [PubMed]
- Schwalm S, Ward RP, Spencer KT. Transient ischemic attack in a patient with pulmonary vein thrombosis after left upper lobectomy for squamous cell lung cancer. J Am Soc Echocardiogr 2004;17:487-8. [Crossref] [PubMed]
- Burri E, Duwe J, Kull C, et al. Pulmonary vein thrombosis after lower lobectomy of the left lung. J Cardiovasc Surg (Torino) 2006;47:609-12. [PubMed]
- Nagaoka E, Yano M, Sugano T, et al. Thrombus in the left superior pulmonary vein after left upper pulmonary lobectomy. J Thorac Cardiovasc Surg 2008;135:709-10. [Crossref] [PubMed]
- Asteriou C, Barbetakis N, Efstathiou A, et al. Renal Artery Thrombosis following Lobectomy for Lung Cancer. Case Rep Oncol 2010;3:208-11. [Crossref] [PubMed]
- Malm B, Hull S, Jadbabaie F. Left upper pulmonary vein thrombus in a patient with atrial fibrillation and prior lobectomy. Am J Med 2014;127:e7-8. [Crossref] [PubMed]
- Gual-Capllonch F, Teis A, Palomeras E. Pulmonary vein spontaneous echocontrast and stroke after pulmonary lobectomy. J Clin Ultrasound 2013;41:321-2. [Crossref] [PubMed]
- Ohira S, Doi K, Okawa K, et al. Surgical removal of extensive left pulmonary vein stump thrombus after pulmonary lobectomy: a rare cause of acute cerebral embolism. Ann Thorac Surg 2013;96:e135-6. [Crossref] [PubMed]
- Asai K, Mochizuki T, Iizuka S, et al. Pulmonary vein stump thrombus: an early complication following upper division segmentectomy of the left lung. Gen Thorac Cardiovasc Surg 2014;62:244-7. [Crossref] [PubMed]
- Ichimura H, Ozawa Y, Nishina H, et al. Thrombus formation in the pulmonary vein stump after left upper lobectomy: a report of four cases. Ann Thorac Cardiovasc Surg 2014;20 Suppl:613-6. [Crossref] [PubMed]
- Wada H, Kobayashi T, Abe Y, et al. Elevated levels of soluble fibrin or D-dimer indicate high risk of thrombosis. J Thromb Haemost 2006;4:1253-8. [Crossref] [PubMed]
- Kodama J, Seki N, Masahiro S, et al. D-dimer level as a risk factor for postoperative venous thromboembolism in Japanese women with gynecologic cancer. Ann Oncol 2010;21:1651-6. [Crossref] [PubMed]
- Dindo D, Breitenstein S, Hahnloser D, et al. Kinetics of D-dimer after general surgery. Blood Coagul Fibrinolysis 2009;20:347-52. [Crossref] [PubMed]
- Yamamoto T, Suzuki H, Nagato K, et al. Is left upper lobectomy for lung cancer a risk factor for cerebral infarction? Surg Today 2016;46:780-4. [Crossref] [PubMed]
- Bateman BT, Schumacher HC, Wang S, et al. Perioperative acute ischemic stroke in noncardiac and nonvascular surgery: incidence, risk factors, and outcomes. Anesthesiology 2009;110:231-8. [PubMed]


