Bronchopulmonary carcinoid with a single lymph node metastasis causing ectopic Cushing’s syndrome
Introduction
Well-differentiated pulmonary neuroendocrine tumors (PNETs) have the ability to excrete neuropeptides, such as ACTH (1). In 15% of cases of Cushing’s syndrome (CS), the source of excess cortisol is an extrapituitary malignancy (2). One to five percent of all bronchial carcinoid tumors (BCTs) produce ectopic ACTH and can present as CS (3).
Case presentation
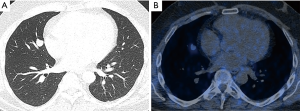
A 39-year-old never smoking male presented with a 6-month history of worsening muscle weakness, fatigue, rest tremor, and increased impulsiveness (beginning of symptoms November 2013). He further complained about recent trunk weight gain, the formation of violaceous striae, and he was diagnosed for arterial hypertension stage 1 (May, 2014). Subsequently, a Dexamethasone-suppression test was performed and revealed a lack of natural adrenocorticotropic hormone (ACTH)-suppression confirming the presence of Cushing’s disease. In August of the same year, a MRI of the head displayed a reduced contrast medium uptake in the pituitary gland covering an area of 2 mm. A thoracic CT-scan (September 2014) showed a lesion in the pulmonary middle lobe measuring 1 cm × 1.5 cm × 1.5 cm without enlargement of thoracic lymph nodes (Figure 1A). Due to these clinical-radiologic findings, an ectopic CS caused by a BCT was suspected. A thoracic PET-CT scan with 68Gallium (somatostatin analog) (October 2014) displayed a mildly Somatostatin-Receptor 2 (SSTR2) positive tumor in the middle lobe, suggesting ACTH production (Figure 1B). Thoracoscopic resection of the middle lobe and lymphadenectomy (ten lymph nodes from station 2, 4, 7, 10, and 12) was performed (October 2014) and histology revealed a typical carcinoid (<1 mitosis per 10 HPF, without necroses) measuring 1.9 cm with infiltration of the visceral pleura. Surprisingly, one out of ten resected lymph nodes revealed a metastasis (lymph node from the middle lobe bronchus, Nr. 12). The immunohistochemistry was positive for Synaptophysin, Keratin, also Pan-Keratin showing a typical patchy cytoplasmatic distribution pattern. The proliferation index was less than 2% resulting in a tumor classification of pT2a, pN1 (1/10). One day after tumor removal, serological values of ACTH and cortisol dropped to almost normal levels (Figure 2). At day of discharge, the patient had already weight loss, improved strength and showed less impulsiveness. Specifically, the patient’s ability to fully work improved to 40% at three months, 60% at 6 months and 100% at 8 months postoperatively).

At 1-year follow up, the patient had fully recovered from all complains, had lost 10 kg of weight, discontinued his antihypertensive medication and hydrocortisone as secondary renal insufficiency subsided and morning cortisol levels restored to normal levels.
Discussion
BCTs are rare, accounting for only a maximum of 5% of all lung tumors. Only an estimated 1–5% of them secret ACTH (1). Typical BCTs rarely metastasize and they are defined as low-grade malignancies. Typical BCTs are described to display different clinical behavior among the subsets of ACTH-secreting BCTs. Beside, these tumors are associated with serious morbidity and a higher mortality risk when compared to their hormone-inactive counterpart (4). Patients with ACTH-producing typical BCTs present with symptoms related to CS, which can be severe and of rapid onset (2).
The early detection of ACTH-producing tissue remains challenging mainly due to a lack of validated imaging modalities (2). While the usage of 68Gallium-PET scan is recommended for those tumors (5), it failed to detect lymph node metastasis in our case. This could be due to the size threshold below 1 cm (1). The time period between diagnosis and eventual resection of the ACTH-secreting BCT can take up to 31 months (3). In case the ectopic ACTH-producing tumor was primarily not detected, an unnecessary hypophysectomy was performed in 27% of cases (1).
This case should alert for the necessity of a meticulous work up upon presentation with CS for ruling out a secondary ectopic ACTH-producing tumor. Although typical BCT rarely metastasize into regional lymph nodes, the fact that ACTH is produced might suggest a correlation with the level of aggressiveness. In these cases, surgery should regularly include lobectomy and lymphadenectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Dincer HE, Podgaetz E, Andrade RS. Pulmonary Neuroendocrine Tumors: Part II. Treatment. J Bronchology Interv Pulmonol 2015;22:351-6. [Crossref] [PubMed]
- Menezes Nunes J, Pinho E, Camões I, et al. A challenging case of an ectopic cushing syndrome. Case Rep Med 2014;2014:413136.
- Boddaert G, Grand B, Le Pimpec-Barthes F, et al. Bronchial carcinoid tumors causing Cushing's syndrome: more aggressive behavior and the need for early diagnosis. Ann Thorac Surg 2012;94:1823-9. [Crossref] [PubMed]
- Lococo F, Margaritora S, Cardillo G, et al. Bronchopulmonary Carcinoids causing Cushing Syndrome: Results from a Multicentric Study Suggesting a More Aggressive Behavior. Thorac Cardiovasc Surg 2016;64:172-81. [PubMed]
- Santhanam P, Taieb D, Giovanella L, et al. PET imaging in ectopic Cushing syndrome: a systematic review. Endocrine 2015;50:297-305. [Crossref] [PubMed]


