Anti-PD-1/PD-L1 antibody versus conventional chemotherapy for previously-treated, advanced non-small-cell lung cancer: a meta-analysis of randomized controlled trials
Introduction
Non-small-cell lung cancer (NSCLC) remains the leading cause of cancer-related death worldwide (1,2). Although advances in chemotherapy and targeted therapy have improved the outcome of metastatic NSCLC, its prognosis remains dismal (3). Effective options are limited for patients with NSCLC whose disease progresses after conventional chemotherapy.
In the last years, immunotherapy is a new strategy for the treatment of previously-treated, advanced NSCLC. Immune checkpoint inhibitors have been shown highly active in different malignancies (4). Targeting the programmed death 1 (PD-1) receptor and its ligand programmed death ligand 1 (PD-L1) pathway is a promising therapeutic option. Recently, the anti-PD-1/PD-L1 monoclonal antibody has been developed for cancer immunotherapy. Emerging evidences have implied that it improves survival in NSCLC patients, thus providing a new treatment option in this setting (5).
Several randomized controlled trials (RCTs) have been performed to investigate the effect of the anti-PD-1/PD-L1 antibody in previously-treated, advanced NSCLC patients. However, only on a limited scale. Performing meta-analyses combining these data could provide a more reliable power to notably assess the value of anti-PD-1/PD-L1 antibody in NSCLC.
We conducted a systematic review and meta-analysis to provide a more reliable and up-to-date evidence on the effect of anti-PD-1/PD-L1 antibody on survival and other key outcomes when compared with chemotherapy.
Methods
Literature-search strategy
A literature search was performed up to May 10, 2016 for published articles using the electronic databases of PubMed, Web of Science, the Cochrane Library and clinicaltrial.gov. Searches were limited to human studies, without language restriction. The following terms and their combinations were searched in [Title/Abstract]: PD-1/PD-L1/Nivolumab/Pembrolizumab/MK-3475/Pidilizumab/MPDL3280A/BMS-936559, non-small-cell lung, cancer/carcinoma, and randomized controlled trial. We also performed manual searches of references cited in the retrieved articles and preceding reviews on the topic. Besides, we reviewed the meeting abstracts and virtual presentations of the American Society of Clinical Oncology annual meetings and European Society of Medical Oncology congresses from 2010 to 2016.
Inclusion and exclusion criteria
Qualified studies meeting the following eligibility criteria were included: RCTs; studies involving patients with previously-treated, advanced NSCLC, which defined as inoperable locally advanced (stage IIIB) or metastatic or recurrent disease (stage IV); studies comparing anti-PD-1/PD-L1 antibody with a conventional chemotherapy agent.
Studies were excluded based on the following criteria: non-human studies; duplicate publications; reported incomplete, useless data; meta-analyses, letters, reviews, or editorial articles.
Data extraction and outcomes interest
Data from the included studies were extracted and summarized independently by two reviewers (Yongxun Zhuansun, Fengting Huang). Disagreement was resolved by the discussion among the authors. The following information was extracted from each article: first author name, year of publication, name of the study, previous chemotherapy agent, pathology or histology of cancer, experiment drug, ECOG status, median age, number of patients with anti-PD-1/PD-L1 antibody treatment or chemotherapy and the follow-up duration.
The primary outcome measure was overall survival (OS) in the intention-to-treat population. The secondary outcomes were: progression-free survival (PFS) in the intention-to-treat population, objective response rate (ORR), the incidence of adverse events, OS and PFS in different PD-L1 expression subgroups.
Quality assessment and statistical analysis
The methodological quality of trials was assessed by the Cochrane risk of bias toll. All the meta-analyses were performed using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK). We addressed time-to-event outcomes by gathering and summarizing hazard ratios (HR) from Cox proportional-hazards models, including for the primary outcome (OS) and secondary survival outcomes. We used the method described by Tierney (6) to calculate HR and/or associated statistics from published time-to-event-analyses when data was not available in the report. The generic inverse-variance method was conducted to pool data where feasible. Pooled dichotomous data from other secondary outcomes was presented as risk ratios. Statistical heterogeneity between studies was assessed using the chi-square test with significance set at P<0.10, and heterogeneity was quantified using the Ι2 statistic. The random-effects model was used if there was heterogeneity between studies; otherwise, the fixed-effects model was used.
Results
Characteristics of included studies
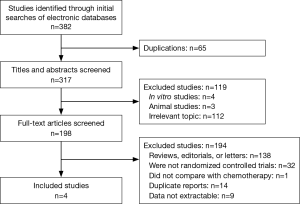
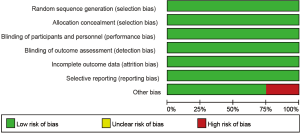
Four studies including 2,174 cases fulfilled the predefined inclusion criteria and were included in the final analysis (Figure 1). The characteristics of included studies are shown in Table 1. All the trials included were of high quality with low bias of selection, performance, detection, attrition and reporting (Figure 2). The patients recruited were histologically confirmed and previously-treated, advanced NSCLC patients. And all trials were open-labeled.
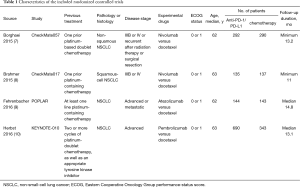
Full table
The outcomes of OS, PFS and ORR in the intention-to-treat population
Anti-PD-1/PD-L1 antibody significantly improved OS compared with chemotherapy (HR 0.67; 95% CI: 0.61–0.75, P<0.00001) (Figure 3). PFS also favored anti-PD-1/PD-L1 antibody (HR 0.81, 95% CI: 0.70–0.95, P=0.009) (Figure 4). The ORR was markedly higher with anti-PD-1/PD-L1 antibody than with chemotherapy (RR of nonresponse, 0.92, 95% CI: 0.89–0.95, P<0.00001) (Figure 5).


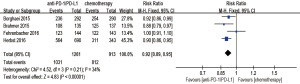
Subgroup analyses
We scored tumor cells expressing PD-L1 as a percentage of total tumor cells (TC) and tumor-infiltrating immune cells expressing PD-L1 as a percentage of tumor area (IC).
Anti-PD-1/PD-L1 antibody treatment predicted a better outcome in evaluating OS when compared with chemotherapy in the TC ≥50% or IC ≥10% (if IC was detected) population (HR 0.56; 95% CI: 0.43–0.72, P<0.00001), the TC ≥5% or IC ≥5% (if IC was detected) population (HR 0.48; 95% CI: 0.37–0.62, P<0.00001), and the TC ≥1% or IC ≥1% (if IC was detected) population (HR 0.64; 95% CI: 0.56–0.73, P<0.00001). However, for the TC <1% and IC <1% (if IC was detected) population, there was no statistic beneficial between anti-PD-1/PD-L1 antibody and chemotherapy in evaluating OS (HR 0.83; 95% CI: 0.66–1.04, P=0.11) (Figure 6).
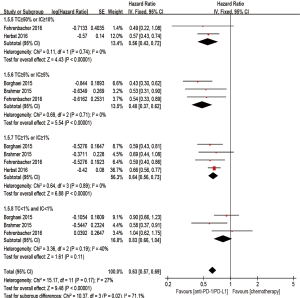
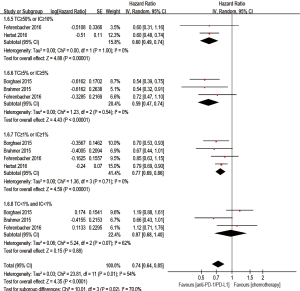
Besides, when compared with chemotherapy, anti-PD-1/PD-L1 antibody showed superiority on PFS in the TC ≥50% or IC ≥10% (if IC was detected) population (HR 0.60; 95% CI: 0.49–0.74, P<0.00001), the TC ≥5% or IC ≥5% (if IC was detected) population (HR 0.59; 95% CI: 0.47–0.74, P<0.00001), and the TC ≥1% or IC ≥1% (if IC was detected) population (HR 0.77; 95% CI: 0.69–0.86, P<0.00001). However, there was no statistic improvement on PFS between the groups in the TC <1% and IC <1% (if IC was detected) population (HR 0.97; 95% CI: 0.68–1.40, P=0.88) (Figure 7).
Safety outcomes
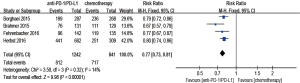
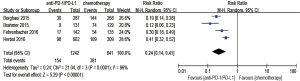
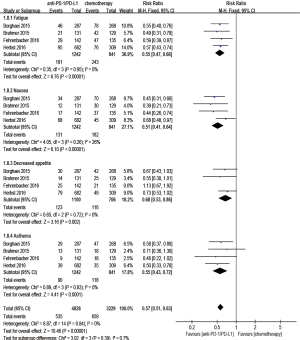
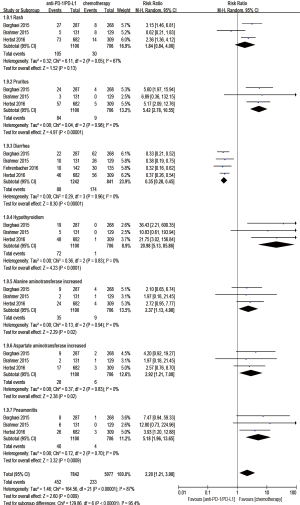
Regarding treatment-related adverse events, the group undergoing anti-PD-1/PD-L1 antibody therapy had less incidence of treatment-related adverse events in any grade (RR 0.77; 95% CI: 0.73–0.81, P<0.00001) (Figure 8), especially in grade 3–5 (RR 0.24; 95% CI: 0.14–0.41, P<0.00001) (Figure 9). The most frequently reported treatment-related adverse events in two groups were fatigue, nausea, decreased appetite and asthenia, which were lower in frequency in anti-PD-1/PD-L1 group than chemotherapy group (Figure 10). The most common adverse events of special interest based on their likely immune etiology in the anti-PD-1/PD-L1 group were rash, pruritus, diarrhea, hypothyroidism, alanine aminotransferase increased, aspartate aminotransferase increased, and pneumonitis, which were higher in frequency in anti-PD-1/PD-L1 group than chemotherapy, except for diarrhea and rash (Figure 11).
Publication bias
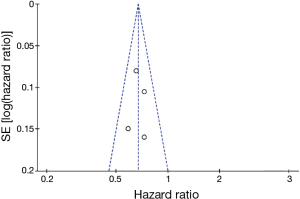
Figure 12 shows a funnel plot of the studies included in this meta-analysis that reported OS. The graphical funnel plots of the studies included appeared to be symmetrical, no evidence of publication bias was detected.
Discussion
This meta-analysis of four RCTs including 2,174 patients indicated that anti-PD-1/PD-L1 antibody could significantly improve OS and PFS, comparing with chemotherapy in previously-treated, advanced NSCLC patients. Also, there was a superior ORR and safety profile in anti-PD-1/PD-L1 antibody treatment.
Antibodies directed against the immunosuppressive molecules PD-1 and PD-L1 have shown remarkable antitumor activity in NSCLC in various of clinical trials (11-13). In the initial phase I-II single arm trials with anti-PD-1/PD-L1 antibodies, durable responses and disease stabilization were reported in patients with NSCLC (14,15). In recent years, several RCTs have been conducted to evaluate the efficiency of anti-PD-1/PD-L1 antibodies in previously-treated, advanced NSCLC patients when compared with chemotherapy, with OS as the primary endpoint (7-10). Docetaxel served as the standard of care, and these four trials included in this meta-analysis all used docetaxel as the comparator. Our data indicated that anti-PD-1/PD-L1 antibody possessed a significant survival benefit over chemotherapy (33% lower risk of death). The OS benefit observed in our meta-analysis is consistent with the results of prior studies of anti-PD-1/PD-L1.
Moreover, our results implied that anti-PD-1/PD-L1 antibody was associated with a significant improvement in PFS (19% lower risk of progression). The benefit of PFS is controversial. A meta-analysis including three RCTs did not show significant improvement of PFS in anti-PD-1/PD-L1 antibody group compared with docetaxel group (16). Notably, our data with more participants and lower publication bias provided a more reliable evidence on this issue. Small population size in individual studies and different inclusion criterion may lead to this inconsistency. Whereas there were separate nivolumab studies for squamous and non-squamous histology, KEYNOTE-010 and POPLAR enrolled patients regardless of histology. Both checkmate studies limited enrolment to who received only one line of previous treatment, whereas KEYNOTE-010 and POPLAR enrolled patients who received at least one line of previous treatment (7-10). Our findings including four RCTs and 2,174 participants provided additional insights into the efficiency of anti-PD-1/PD-L1 to improve PFS. However, this issue needs to be reevaluated in large RCTs in the future. The benefit of anti-PD-1/PD-L1 antibody was further reflected by a significantly higher ORR as compared with chemotherapy (RR of nonresponse, 0.92).
Identifying patients most likely to benefit from anti-PD-1/PD-L1 antibody is a clinical challenge worthy of more attention. In our meta-analysis, improvements in OS and PFS were observed in the patients with TC or IC >1%. It is implied that anti-PD-1/PD-L1 antibody treatment may contribute to a better outcome in the patients with TC or IC >1%.
The safety profile of anti-PD-1/PD-L1 antibody was favorable in comparison with chemotherapy, with less treatment-related adverse events of any grade, especially of grade 3–5. Immune-mediated adverse events with immunotherapies such as pneumonitis and hypothyroidism were much higher in frequencies than that of chemotherapy. However, these adverse events were always infrequent and of low severity and were managed with the use of established guidelines.
The present meta-analysis has the following limitation that must be taken into account. Four RCTs were included in the meta-analysis. KEYNOTE-010 was a large study that included a high percentage of the patients included in our analysis. An eligibility inclusion criterion for KEYNOTE-010 was that the tumors have at least 1% tumor cells positive for PD-L1 in contrast to CheckMate 017, CheckMate 057, and the POPLAR study that included both PD-L1 positive and negative. This creates a bias in the data for enrichment of tumors that are PD-L1 positive. High levels PD-L1 expression on tumors correlates with response to anti-PD-1/PD-L1 antibody (17). Therefore, the enrichment for PD-L1 positive tumors creates bias to overestimate the efficiency of anti-PD-1/PD-L1 antibody in the entire group that combines PD-L1 positive and negative. Besides, in the subgroup analyses, anti-PD-1/PD-L1 antibody did not show survival benefit over chemotherapy among patients whose tumors did not express PD-L1. This indicates that anti-PD-1/PD-L1 antibody must be carefully evaluated for tumors that are PD-L1 negative.
In conclusion, anti-PD-1/PD-L1 antibody had a better safety profile and superior survival benefit over chemotherapy in patients with previously-treated, advanced NSCLC.
Acknowledgements
We thank doctor Xinxiang Fan for the instruction of this meta-analysis.
Funding: This work was supported by grants from Major program of Science and Technology Program of Guangzhou, China [201604020103]; National Natural Science Foundation of Guangdong, China [2015A030313134]; and Medical Scientific Research Foundation of Guangdong Province, China [B2014128].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Hirsch FR, Suda K, Wiens J, et al. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 2016;388:1012-24. [Crossref] [PubMed]
- Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol 2013;94:41-53. [Crossref] [PubMed]
- Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Zhou GW, Xiong Y, Chen S, et al. Anti-PD-1/PD-L1 antibody therapy for pretreated advanced nonsmall-cell lung cancer: A meta-analysis of randomized clinical trials. Medicine (Baltimore) 2016;95:e4611. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]