Efficacy of nebulized colistin-based therapy without concurrent intravenous colistin for ventilator-associated pneumonia caused by carbapenem-resistant Acinetobacter baumannii
Introduction
Carbapenem resistance in Acinetobacter baumannii(CRAB) infection has become increasingly prevalent worldwide and has been associated with high mortality rate (1-3). Patients with ventilator-associated pneumonia (VAP) caused by carbapenem-resistant CRAB, the incidence of which has been rising substantially, may be predisposed to poor outcome because of limited therapeutic options and the likelihood of inappropriate empirical antibiotic therapy (4,5).
Colistin (polymyxin E) is one of the few agents that can be used for treatment of CRAB infections, and there is considerable evidence regarding the efficacy of colistin for VAP caused by CRAB (6,7). In addition, recent studies revealed that nebulized colistin, as adjunctive therapy simultaneously administered with intravenous colistin, was significantly associated with improved rate of clinical cure and reduced duration of mechanical ventilation without noticeable adverse events in patients with VAP due to CRAB (8,9). The use of nebulized colistin in critically ill patients may be supported by previous reports suggesting that it effectively achieved high concentrations in the lungs, with minimal systemic exposure (10,11). However, there is a paucity of data to evaluate the efficacy of nebulized colistin as monotherapy for pneumonia of multidrug-resistant gram-negative bacteria (12-14). Exploration of this approach is worthwhile because acute kidney injury (AKI) during intravenous colistin therapy remains a great concern, particularly in elderly patients in intensive care units (ICU) with impaired renal function and concomitant use of other nephrotoxic agents (15,16).
In this study, we present our experience in using nebulized colistin, without concurrent intravenous colistin, in the treatment of VAP caused by CRAB. The aim of our current study was to compare the clinical outcomes of VAP caused by CRAB treated with nebulized colistin vs. intravenous colistin. A further objective was to assess the optimal use of nebulized colistin to improve outcomes.
Methods
Study design and population
This retrospective study was conducted at Inje University Haeundae Paik Hospital and Inje University Busan Paik Hospital, 1,000-bed and 900-bed university-affiliated hospitals, respectively, in Busan, Korea. We reviewed the medical charts of patients admitted to the medical or surgical ICU between March 2010 and November 2015.
Eligibility criteria were as follows: (I) adult patients (≥18 years of age) who were diagnosed with pneumonia defined as a new or progressive pulmonary infiltrates on chest radiograph with at least two findings of fever >38 °C or hypothermia <35.5 °C with no other identified cause, leukocytosis (white blood cells ≥12,000×103/L) or leukopenia (white blood cells <4,000×103/L), purulent tracheal secretions, a decrease in oxygenation (17); (II) culture-documented monomicrobial VAP caused by CRAB with onset (the date of the index culture study) after ≥48 h of mechanical ventilation (18); (III) positive results of CRAB cultures from at least two sets of tracheobronchial secretions and/or one sample of bronchoalveolar lavage (BAL) fluid; (IV) intravenous or nebulized colistin administered for ≥3 days and initiated within a period of 5 days before or after the date of index culture study. Patients who had concurrent CRAB bacteremia and/or received both nebulized and intravenous colistin simultaneously were excluded.
Data collection and definitions
The acute physiology and chronic health evaluation (APACHE) II score on the day of VAP onset that coincided with the collection date of the index culture study was calculated. Clinical Pulmonary Infection Score (CPIS) with a range of 0 to 12 was used for the diagnosis of VAP (19). The severity of sepsis was graded using the American College of Chest Physicians/Society of Critical Care Medicine consensus criteria (20).
Immunosuppressive therapy was defined as use of corticosteroid for at least 10 days, chemotherapy or radiotherapy during the last 30 days, or other recognized T cell immunosuppressants such as TNF-α blockers and calcineurin inhibitors during the last 30 days. Empirical therapy was considered as appropriate if at least one susceptible antibiotic against CRAB was administered during initial therapy, and combination therapy with colistin was defined as at least 3 days of concomitant use of other antibiotics.
Clinical failure was defined as persistence or worsening of signs or symptoms of pneumonia and lack of improvement of radiologic pulmonary infiltrates. Clinical outcomes were assessed at the end of colistin therapy or at the time of discharge from ICU, whichever was earlier. Data was independently reviewed by one physician in the Division of Infectious Diseases (Y.K.K) and two physicians in the Division of Pulmonology and Critical Care Medicine (J.H.L and H.Y.L). To avoid inaccurate decisions regarding clinical outcomes, cases which initially had conflicting results of interpretation between reviewers were classified as indeterminate. The cases initially classified as indeterminate were discussed in a conference, and a consensus was reached by the reviewers who were not aware of patients’ therapy group.
Microbiological failure was considered if at least two consecutive cultures from tracheobronchial secretion specimens and/or at least one from BAL fluid specimen had failed to reveal no growth of CRAB by the end of colistin therapy. If regrowth of CRAB during colistin therapy was observed after at least two negative results of culture, it was also classified as microbiological failure.
In patients with normal renal function, AKI was defined as a serum creatinine level >2 mg/dL, or a ≥50% decrease in the glomerular filtration rate compared with the initial value at the start of treatment, or a deterioration of renal function that needed renal replacement therapy (8). In patients with preexisting renal dysfunction, AKI was defined as >50% of the baseline serum creatinine or a reduction in the calculated creatinine clearance of 50% compared with the initial value (8).
Microbiological studies and treatment regimens
Semiquantitative cultures (moderate or heavy growth) of tracheobronchial specimens with the presence of >25 neutrophils, 10< epithelial cells per low-power field with compatible gram stain findings and BAL specimens are required to define the causative CRAB isolates. The results of in vitro susceptibility tests were interpreted according to the Clinical Laboratory Standards Institute (CLSI) guidelines (21). CRAB isolates were considered susceptible to colistin and tigecycline if the minimum inhibitory concentrations (MICs) ≤2 mg/L were observed in the broth microdilution method.
The daily dose of nebulized colistin colistimethate sodium (CMS) ranged from 75 mg colistin base activity (CBA) every 12 hours to 150 mg CBA every 8 hours, and each dose was diluted in 5 mL sterile normal saline. It was administered over 30 min via a conventional jet nebulizer connected to the inspiratory limb of ventilator circuit (Sileo 54, Macjin Medical). Bronchodilator, such as salbutamol, was routinely used 30 minutes prior to administration of nebulized colistin. In terms of CBA, the dose of intravenous colistin was 5 mg/kg/day (divided into 2 doses) in patients with creatinine clearance of ≥80 mL/min, 2.5–3.8 mg/kg/day (divided into 2 doses) in patients with creatinine clearance ranging from 50 to 80 mL/min. Intravenous colistin of 2–2.5 mg/kg/day (divided into 2 doses) or 1–1.5 mg/kg every 36 hours was administered in patients with creatinine clearance ranging from 30 to 50 mL/min or from 10 to 30 mL/min, respectively. No initial loading dose of intravenous colistin was used.
Statistical analysis
All statistical analyses were performed using SPSS for Windows software package, version 21 (SPSS Inc., Chicago, IL, USA). Categorical variables were compared using the χ2 or Fisher’s exact test, and continuous variables were compared using the Mann-Whitney U test, as appropriate. All tests of significance were two-tailed and a P<0.05 was considered to indicate statistical significance. The propensity score for the probability of treatment with nebulized or intravenous colistin was estimated using a logistic regression using baseline and clinical characteristics listed in Table 1. Variables considered independent for the propensity scores included age, chronic kidney disease, medical or trauma-related ICU admission, APACHE II score, septic shock, colistin monotherapy, carbapenem use, minocycline use, amikacin use. Patients were matched in a 1:1 ratio using nearest neighbor method. To evaluate risk factors of clinical failure in patients after propensity score matched data set, and in a selected group of patients treated with nebulized colistin-based therapy, all variables with P<0.1 in the univariable analysis were included in a multivariable logistic regression model.
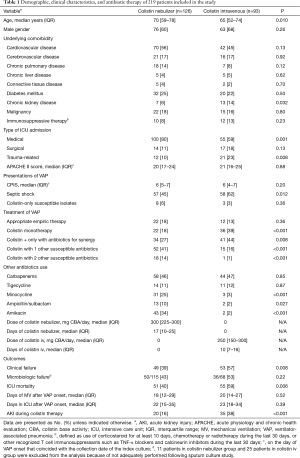
Full table
Results
During the study period, we identified 219 patients who met the eligibility criteria. Of these 219 patients, 126 patients were treated with nebulized colistin and 93 were treated with intravenous colistin (Table 1). Patients in the nebulized colistin group were older; however, they were less likely to have chronic kidney disease (6% vs. 14%; P=0.032). Patients with trauma-related admission were more likely to receive intravenous colistin (23% vs. 10%; P=0.008), and patients who were admitted to medical ICU received nebulized colistin more frequently (80% vs. 59%; P=0.001). Significant differences in severity of sepsis and combination therapy with other antibiotics were observed between the two groups. The nebulized colistin cohort included a smaller number of patients with septic shock (45% vs. 62%, P=0.012), but concomitantly received other susceptible antibiotics against CRAB more frequently (55% vs. 17%, P<0.001). Minocycline and amikacin were the most commonly used agents if they had in vitro susceptibility, and administered significantly more often in the nebulized colistin cohort (25% vs. 3%; P<0.001 and 34% vs. 2%; P<0.001, respectively). All patients treated with colistin-carbapenem combination (imipenem for 41 patients, and meropenem for 61 patients) received carbapenems with intermittent bolus dosing and standard dosage regimens, regardless of severity of sepsis.
Table 1 shows that clinical failure and ICU mortality were significantly associated with use of intravenous colistin (57% vs. 39%; P=0.008 and 59% vs. 40%; P=0.006, respectively). In addition, AKI during colistin therapy was significantly more common in the intravenous colistin cohort (38% vs. 16%; P<0.001). Neither dose reduction nor discontinuation of colistin was observed in 20 patients who developed AKI in nebulized colistin group, and none of patients required renal replacement therapy. In contrast, all 35 patients with AKI during therapy in intravenous colistin group had dose reduction. Nine patients discontinued colistin therapy, and three patients required initiation of renal replacement therapy. Six patients died without recovery of renal function. Bronchospasm, the common side effect of nebulized colistin, was not observed in the study patients, and neurotoxicity was observed only one patient with an episode of seizure in intravenous colistin group. Days of mechanical ventilation and ICU stay after VAP onset did not differ between the two groups (P=0.52 and P=0.39, respectively).
Propensity-score-matched analysis
Of 219 patients, 39 patients in each group were matched by propensity score. Table 2 shows comparisons of demographic, clinical characteristics, and antibiotic therapy between the propensity score-matched groups. There were no significant differences in baseline and clinical characteristics, such as chronic kidney disease (5% vs. 8%, P>0.99), medical ICU admission (72% vs. 72%, P>0.99), septic shock (56% vs. 54%, P=0.82), concurrent active systemic antibiotics, between the matched groups. There were no significant differences in the rates of clinical failure between the two groups [Odds ratio (OR), 0.48; 95% confidence interval (CI), 0.19–1.19; P=0.11]. However, a rate of AKI during colistin therapy was significantly lower in nebulized colistin group (18% vs. 49%, P=0.004).
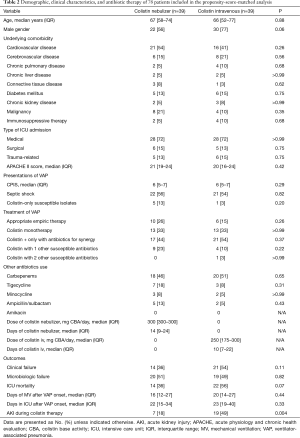
Full table
The effect of nebulized colistin on clinical failure and the risk factors associated with clinical failure were analyzed in the propensity score-matched set (Table 3). In multivariable analysis, there was no significant difference in clinical failure between the two groups [adjusted odds ratio (aOR), 0.36; 95% CI, 0.12–1.09; P=0.070]. The independent risk factors for clinical failure were medical ICU admission (aOR, 7.14; 95% CI, 1.60–32.00; P=0.010), and septic shock (aOR, 3.93; 95% CI, 1.27–12.17; P=0.018).
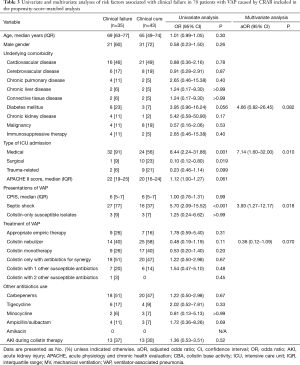
Full table
Risk factors for clinical failure in subgroup of nebulized colistin cohort
Table 4 shows the analysis of risk factors for clinical failure in a selected group of patients treated with nebulized colistin. In multivariable analysis performed with variables with P<0.1 as determined using univariable analysis, connective tissue disease (P=0.039), septic shock (P=0.002), combination therapy with carbapenem (P=0.011), and AKI during colistin therapy (P=0.028) were significantly associated with clinical failure.
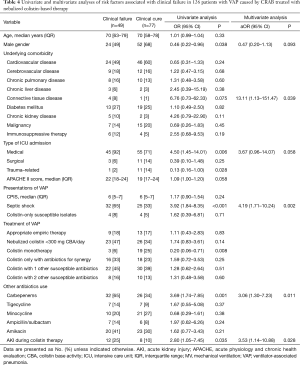
Full table
In terms of doses of nebulized colistin, despite the lack of statistical significance, univariable analysis revealed that doses of nebulized colistin less than 300 mg CBA/day showed a tendency toward an increased rate of clinical failure (OR, 1.74; 95% CI, 0.83–3.61; P=0.14).
Discussion
In the present study, we reviewed patients with VAP due to CRAB treated with either intravenous or nebulized colistin, and not administered concurrently, over a period of 5 years. Although there have been reports of the efficacy of nebulized colistin as monotherapy for nosocomial pneumonia caused by gram-negative bacteria, the main drawbacks of the previous studies were small number of patients, lack of a control group, and different causative microorganisms (12,13,22,23). One recent study reinforced the efficacy and safety of aerosolized colistin in the treatment of gram-negative VAP (24). However, in that study, the causative organisms are various, and patients with septic shock were excluded. To the best of our knowledge, this is the largest study that included patients treated with nebulized colistin without concurrent intravenous colistin in the treatment of VAP caused by CRAB. Notably, nebulized colistin in combination with other susceptible antibiotics showed comparable efficacy to intravenous colistin. In addition, nebulized colistin was associated with a lower rate of AKI during therapy, although its effect on clinical failure was not significant in our study.
The optimal treatment for VAP due to CRAB is still controversial. Colistin is usually recommended as a first-line agent based on proven efficacy and safety (6,7,25,26). Recent meta-analyses showed the benefits of nebulized colistin for VAP caused by multidrug-resistant pathogens; however, the role of this form of colistin might be adjunctive to intravenous colistin (27-29). We posit that the use of nebulized colistin can be supported by pharmacokinetic-pharmacodynamic (PK-PD) data regarding antimicrobial efficacy and delivery to the lungs (10,11,30), and that our present study has important implications which suggests that nebulized colistin may be used for VAP caused by CRAB, even without concurrent intravenous administration. However, nebulized colistin should be considered to be used with other systemic antibiotics active against CRAB isolates until further well-designed clinical trials based on randomization confirm the efficacy of nebulized colistin monotherapy in the treatment of VAP due to CRAB. In addition, cautious interpretation of the results in patients with septic shock should be applied because only four patients received nebulized colistin monotherapy and 50% (2/4) of patients died.
One of the important findings of our study is that the dosing regimen of nebulized colistin should be intensified because of the presumed survival benefit of a higher inhaled dose. Although there are limited data on appropriate dosing regimens of nebulized colistin to enhance treatment outcomes, recent studies suggest that a sufficient inhalation dose, of at least a total daily dose of 480 mg CMS, should be recommended for severe pulmonary infections, which may be reinforced by our data (26,31). Furthermore, the results of our study support previous pharmacokinetic data that higher doses of inhaled colistin were required for better effectiveness in critically ill patients (10). From our point of view, further studies to identify the optimal inhaled doses of colistin and overcome the heterogeneity of practice would help clinicians treat VAP due to CRAB more effectively (32).
Another important finding of our study was that the nebulized colistin group had a significantly lower rate of nephrotoxicity in both overall patients and propensity-score-matched patients, which was consistent with other studies (29). Interestingly, however, AKI developed in 16% (20/126) of patients with nebulized colistin. A potential explanation for this may be that nebulized colistin was coadministered with other nephrotoxic drugs, such as amikacin and nonsteroidal anti-inflammatory drugs (NSAIDs), in 80% (16/20) of patients. In addition, there were two patients with baseline renal impairment who developed AKI during nebulized colistin therapy. Although AKI during therapy did not significantly affect rates of clinical failure in our study, there have been conflicting results on the influence of AKI on mortality (33,34). From our point of view, there remain concerns about the use of nebulized colistin in patients with either decreased renal function or concomitant other nephrotoxic agents because AKI during therapy may be associated with poor outcomes. Therefore, caution is needed until more clinical trials and pharmacokinetic evaluation of nebulized colistin validate its safety in such conditions.
Efficacy of antimicrobial combination therapy, compared with monotherapy, against VAP caused by CRAB is another issue of great interest in this study. Despite some promising results for combination therapy and synergistic interaction between colistin and other antibiotics (35,36), the clinical cure rate in our data did not differ whether or not other antibiotics were used with colistin. Notably, nebulized colistin-carbapenem combination led to worse outcomes in subgroup analysis of this study. The results might be attributable to insufficient concentrations or suboptimal methods of administration of the carbapenems in patients with septic shock. A recent study by Khawcharoenporn et al. showed that combination therapy of nebulized colistin and carbapenems was an effective treatment option if carbapenems were given in a prolonged infusion fashion (37). In addition, previous studies indicated that individualized dosing of β-lactam antibiotics should be considered to increase the likelihood of optimal outcomes because of altered pharmacokinetics of β-lactam in critically ill patients (38,39). Because all patients in this study treated with colistin-carbapenem combination therapy received standard dosage regimen for carbapenems with intermittent bolus dosing, the unexpectedly low efficacy of colistin-carbapenem combination might occur. Furthermore, relatively low rate of synergy of imipenem and the effect of CRAB strains showing a high MIC for meropenem (MIC ≥64 mg/L) might have influenced the overall results (40-42). The possibility of unexpected antagonistic effect and suboptimal doses of other antibiotics should lead to a cautious interpretation of the results on combination therapy (43,44). Taken together, a multidisciplinary approach by physicians and microbiologists to perform routine synergy tests to determine the appropriate combination therapy should be considered. In addition, colistin-minocycline combination for treatment of VAP due to CRAB needs consideration based on this present data and previous reports (45,46).
Our study had several limitations. First, the retrospective design of our analyses may have introduced information bias or missed certain changes that influenced the results. Moreover, uneven baseline clinical characteristics of the study patients could also have confounded the results. In addition to systemic data collection and verification, we attempted to adjust for these drawbacks and control the bias in treatment assignment by using propensity-score-matched analysis. Second, different dosage regimen of intravenous colistin without loading dose, compared with currently recommended doses, might have had an influence on clinical outcomes (47). Third, microorganisms other than Acinetobacter baumannii such as carbapenem-resistant Pseudomonas aeruginosa were not considered, and only 11 isolates of colistin-only susceptible Acinetobacter baumannii were included in our analyses. Therefore, our results cannot ensure the consistency in those pathogens. Finally, we could not assess the information on the amount of nebulized colistin delivered to lungs, because we only used conventional ventilator nebulizers in our study patients. Despite these limitations, our study is valuable because of the scarcity of comparative studies that specifically evaluated the efficacy of nebulized colistin, not concurrently administered with intravenous colistin, in cases of VAP due to CRAB. Moreover, our study has strength in having relatively large number of patients and a control group, compared with previous studies.
In conclusion, our findings suggest that nebulized colistin, even without concurrent administration of intravenous colistin, may provide a useful treatment option to clinicians confronted with VAP due to CRAB. However, nebulized colistin needs to be used with other active systemic antibiotics until further evidences based on randomized trials confirm the optimal colistin-based therapy in the treatment of VAP due to CRAB. To further improve treatment outcomes, optimal inhaled doses of colistin and appropriate synergy tests for precise combination therapy should be examined.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Institutional Review Board (IRB) of Inje University Haeundae Paik Hospital (201612012) and Inje University Busan Paik Hospital (20160272).
References
- Perez F, Hujer AM, Hujer KM, et al. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2007;51:3471-84. [Crossref] [PubMed]
- Kang CI, Song JH. Antimicrobial resistance in Asia: current epidemiology and clinical implications. Infect Chemother 2013;45:22-31. [Crossref] [PubMed]
- Lemos EV, de la Hoz FP, Einarson TR, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect 2014;20:416-23. [Crossref] [PubMed]
- Tseng CC, Liu SF, Wang CC, et al. Impact of clinical severity index, infective pathogens, and initial empiric antibiotic use on hospital mortality in patients with ventilator-associated pneumonia. Am J Infect Control 2012;40:648-52. [Crossref] [PubMed]
- Kollef KE, Schramm GE, Wills AR, et al. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest 2008;134:281-7. [Crossref] [PubMed]
- Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 2005;40:1333-41. [Crossref] [PubMed]
- Gu WJ, Wang F, Tang L, et al. Colistin for the treatment of ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: a systematic review and meta-analysis. Int J Antimicrob Agents 2014;44:477-85. [Crossref] [PubMed]
- Kofteridis DP, Alexopoulou C, Valachis A, et al. Aerosolized plus intravenous colistin versus intravenous colistin alone for the treatment of ventilator-associated pneumonia: a matched case-control study. Clin Infect Dis 2010;51:1238-44. [Crossref] [PubMed]
- Tumbarello M, De Pascale G, Trecarichi EM, et al. Effect of aerosolized colistin as adjunctive treatment on the outcomes of microbiologically documented ventilator-associated pneumonia caused by colistin-only susceptible Gram-negative bacteria. Chest 2013;144:1768-75. [Crossref] [PubMed]
- Athanassa ZE, Markantonis SL, Fousteri MZ, et al. Pharmacokinetics of inhaled colistimethate sodium (CMS) in mechanically ventilated critically ill patients. Intensive Care Med 2012;38:1779-86. [Crossref] [PubMed]
- Boisson M, Jacobs M, Grégoire N, et al. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother 2014;58:7331-9. [Crossref] [PubMed]
- Kwa AL, Loh C, Low JG, et al. Nebulized colistin in the treatment of pneumonia due to multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis 2005;41:754-7. [Crossref] [PubMed]
- Falagas ME, Siempos II, Rafailidis PI, et al. Inhaled colistin as monotherapy for multidrug-resistant gram (-) nosocomial pneumonia: a case series. Respir Med 2009;103:707-13. [Crossref] [PubMed]
- Kang CH, Tsai CM, Wu TH, et al. Colistin inhalation monotherapy for ventilator-associated pneumonia of Acinetobacter baumannii in prematurity. Pediatr Pulmonol 2014;49:381-8. [Crossref] [PubMed]
- Balkan II, Dogan M, Durdu B, et al. Colistin nephrotoxicity increases with age. Scand J Infect Dis 2014;46:678-85. [Crossref] [PubMed]
- Lee YJ, Wi YM, Kwon YJ, et al. Association between colistin dose and development of nephrotoxicity. Crit Care Med 2015;43:1187-93. [Crossref] [PubMed]
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309-32. [Crossref] [PubMed]
- American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. [Crossref] [PubMed]
- Pugin J, Auckenthaler R, Mili N, et al. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic "blind" bronchoalveolar lavage fluid. Am Rev Respir Dis 1991;143:1121-9. [Crossref] [PubMed]
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644-55. [Crossref] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-second Informational Supplement M100-S22, 2012. In CLSI, Wayne, PA, USA.
- Ghannam DE, Rodriguez GH, Raad II, et al. Inhaled aminoglycosides in cancer patients with ventilator-associated Gram-negative bacterial pneumonia: safety and feasibility in the era of escalating drug resistance. Eur J Clin Microbiol Infect Dis 2009;28:253-9. [Crossref] [PubMed]
- Naesens R, Vlieghe E, Verbrugghe W, et al. A retrospective observational study on the efficacy of colistin by inhalation as compared to parenteral administration for the treatment of nosocomial pneumonia associated with multidrug-resistant Pseudomonas aeruginosa. BMC Infect Dis 2011;11:317. [Crossref] [PubMed]
- Abdellatif S, Trifi A, Daly F, et al. Efficacy and toxicity of aerosolised colistin in ventilator-associated pneumonia: a prospective, randomised trial. Ann Intensive Care 2016;6:26. [Crossref] [PubMed]
- Florescu DF, Qiu F, McCartan MA, et al. What is the efficacy and safety of colistin for treatment of ventilator-associated pneumonia? A systematic review and meta-regression. Clin Infect Dis 2012;54:670-80. [Crossref] [PubMed]
- Gurjar M. Colistin for lung infection: an update. J Intensive Care 2015;3:3. [Crossref] [PubMed]
- Liu D, Zhang J, Liu HX, et al. Intravenous combined with aerosolised polymyxin versus intravenous polymyxin alone in the treatment of pneumonia caused by multidrug-resistant pathogens: a systematic review and meta-analysis. Int J Antimicrob Agents 2015;46:603-9. [Crossref] [PubMed]
- Zampieri FG, Nassar AP Jr, Gusmao-Flores D, et al. Nebulized antibiotics for ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care 2015;19:150. [Crossref] [PubMed]
- Valachis A, Samonis G, Kofteridis DP. The role of aerosolized colistin in the treatment of ventilator-associated pneumonia: a systematic review and metaanalysis. Crit Care Med 2015;43:527-33. [Crossref] [PubMed]
- W S Yapa S. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: targeting advantage of inhalational administration. Antimicrob Agents Chemother 2014;58:2570-9. [Crossref] [PubMed]
- Lu Q, Luo R, Bodin L, et al. Efficacy of high-dose nebulized colistin in ventilator-associated pneumonia caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Anesthesiology 2012;117:1335-47. [Crossref] [PubMed]
- Solé-Lleonart C, Roberts JA, Chastre J, et al. Global survey on nebulization of antimicrobial agents in mechanically ventilated patients: a call for international guidelines. Clin Microbiol Infect 2016;22:359-64. [Crossref] [PubMed]
- Kwon KH, Oh JY, Yoon YS, et al. Colistin treatment in carbapenem-resistant Acinetobacter baumannii pneumonia patients: incidence of nephrotoxicity and outcomes. Int J Antimicrob Agents 2015;45:605-9. [Crossref] [PubMed]
- Tuon FF, Rigatto MH, Lopes CK, et al. Risk factors for acute kidney injury in patients treated with polymyxin B or colistin methanesulfonate sodium. Int J Antimicrob Agents 2014;43:349-52. [Crossref] [PubMed]
- Shields RK, Kwak EJ, Potoski BA, et al. High mortality rates among solid organ transplant recipients infected with extensively drug-resistant Acinetobacter baumannii: using in vitro antibiotic combination testing to identify the combination of a carbapenem and colistin as an effective treatment regimen. Diagn Microbiol Infect Dis 2011;70:246-52. [Crossref] [PubMed]
- Poulikakos P, Tansarli GS, Falagas ME. Combination antibiotic treatment versus monotherapy for multidrug-resistant, extensively drug-resistant, and pandrug-resistant Acinetobacter infections: a systematic review. Eur J Clin Microbiol Infect Dis 2014;33:1675-85. [Crossref] [PubMed]
- Khawcharoenporn T, Pruetpongpun N, Tiamsak P, et al. Colistin-based treatment for extensively drug-resistant Acinetobacter baumannii pneumonia. Int J Antimicrob Agents 2014;43:378-82. [Crossref] [PubMed]
- Taccone FS, Laterre PF, Dugernier T, et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care 2010;14:R126. [Crossref] [PubMed]
- Ulldemolins M, Vaquer S, Llauradó-Serra M, et al. Beta-lactam dosing in critically ill patients with septic shock and continuous renal replacement therapy. Crit Care 2014;18:227. [Crossref] [PubMed]
- Zusman O, Avni T, Leibovici L, et al. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother 2013;57:5104-11. [Crossref] [PubMed]
- Ni W, Shao X, Di X, et al. In vitro synergy of polymyxins with other antibiotics for Acinetobacter baumannii: a systematic review and meta-analysis. Int J Antimicrob Agents 2015;45:8-18. [Crossref] [PubMed]
- Fan B, Guan J, Wang X, et al. Activity of colistin in combination with meropenem, tigecycline, fosfomycin, fusidic acid, rifampin or sulbactam against extensively drug-resistant Acinetobacter baumannii. PLos One 2016;11:e0157757. [Crossref] [PubMed]
- Park GC, Choi JA, Jang SJ, et al. In vitro interactions of antibiotic combinations of colistin, tigecycline, and doripenem against extensively drug-resistant and multidrug-resistant Acinetobacter baumannii. Ann Lab Med 2016;36:124-30. [Crossref] [PubMed]
- De Pascale G, Antonelli M. Appropriate tigecycline use for extensively drug-resistant infections: The standard dose may not be enough! Crit Care Med 2015;43:e533-4. [Crossref] [PubMed]
- Goff DA, Kaye KS. Minocycline: an old drug for a new bug: multidrug-resistant Acinetobacter baumannii. Clin Infect Dis 2014;59:S365-6. [Crossref] [PubMed]
- Liang W, Liu XF, Huang J, et al. Activities of colistin- and minocycline-based combinations against extensive drug resistant Acinetobacter baumannii isolates from intensive care unit patients. BMC Infect Dis 2011;11:109. [Crossref] [PubMed]
- Nation RL, Garonzik SM, Li J, et al. Updated US and European dose recommendations for intravenous colistin: How do they perform? Clin Infect Dis 2016;62:552-8. [Crossref] [PubMed]


