Age before duty: the effect of storage duration on mortality after red blood cell transfusion
The transfusion of blood products is one of the most commonly implemented therapies in modern medicine. Over 20 million blood components are transfused each year in the United States alone, of which approximately 13 million are packed red blood cell (pRBC) units (1). Current US Food and Drug Administration (FDA) regulations allow storage of pRBC units for up to 42 days prior to transfusion (2). Established in 1985, this blood banking policy reflects erythrocyte function as a measure of post-transfusion survival and storage-related hemolysis (3). Since then, many other metrics of red blood cell deterioration have been elucidated. Collectively termed the red blood cell storage lesion, a host of biochemical, metabolic, and structural changes have been shown to occur within the pRBC unit during the storage period (2,4). Elements of this storage lesion cause harm in animal models of transfusion, suggesting that the transfusion of stored pRBC units may lead to worsened outcomes in human patients and highlighting the need for high quality clinical studies (5-7).
Many clinical studies attempting to address this topic have yielded conflicting results. One reason for this is that these studies have been limited by small sample sizes. Another reason is that the definition of fresh blood and aged blood has been inconsistent across studies (Table 1) (8-21). Several articles have defined fresh blood as a function of time, ranging anywhere from 5 days (8) to 21 days (17), while others have taken a more practical approach, employing a “freshest available” policy (18,20). The definition of aged blood has also suffered from wide variations. Notably, only one study to date has investigated the effect of pRBC units transfused in their last week of storage, observing adverse outcomes in high-risk patients (17).
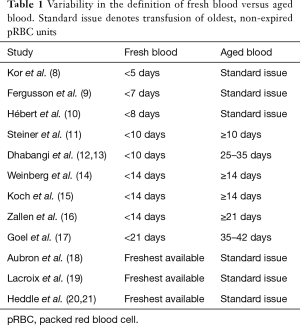
Full table
Recognizing the need for a definitive, high-powered study, Heddle et al. launched a large clinical trial spanning six hospitals across four countries. As published in the New England Journal of Medicine, the Informing Fresh versus Old Red Cell Management (INFORM) trial (21) randomized 31,497 transfusion recipients to pRBC units stored for a “short-” or “long-term” duration. The authors employed a “freshest available” approach to short-term storage, and “standard issue” policy to the long-term storage group. Median storage of pRBC units prior to transfusion was 11 days [interquartile ratio (IQR), 8–15] in the short-term group, as compared to 24 days (IQR 18–30) in the long-term group. There were no differences in in-hospital mortality between the two cohorts. Subgroup analysis of high-risk patient populations also revealed no differences in mortality rates with “long-term” storage blood transfusion (e.g., cardiovascular surgery, critically ill, and cancer patients).
The INFORM trial is the largest randomized study to date investigating the effect of blood storage duration on patient mortality. By utilizing a pilot trial (20), any logistical concerns and questions of feasibility were addressed prior to initiation of the study. Unfortunately, several limitations prevent the INFORM trial from providing a conclusive statement to the aged blood dilemma. First, the current study is not a true comparison between fresh and aged pRBC units, but rather, a comparison of “standard issue” and “freshest available” policies. While the actual definition of aged blood has been a point of contention, a storage period of 24 days falls near the middle of the accepted storage limit. Second, the authors admit that their electronic medical records do not provide information on coexisting illnesses. A compelling analysis of in-hospital mortality would include medical comorbidities and patient severity of illness as covariates in the statistical models. Third, the indications for blood transfusion are unknown. Ideally, mortality would be best compared in patients receiving pRBC units for similar indications.
Given the power of this large trial, it would be interesting to analyze data concerning the rates of adverse transfusion reactions, such as transfusion-associated acute lung injury. Based on current data, one can conclude that standard practice of transfusing oldest available blood is non-inferior to transfusion of freshest available blood with regard to in-hospital mortality. The question of whether aged blood portends worse clinical outcomes, however, remains to be answered.
Acknowledgements
Funding: This work was supported by NIH grants R01 GM107625 (TAP) and T32 GM008478-24 (YK).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Blood Facts and Statistics. American Red Cross 2016. Available online: http://www.redcrossblood.org/learn-about-blood/blood-facts-and-statistics
- Hoehn RS, Jernigan PL, Chang AL, et al. Molecular mechanisms of erythrocyte aging. Biol Chem 2015;396:621-31. [Crossref] [PubMed]
- United States Food and Drug Administration. Workshop on Red Cell Stored in Additive Solution Systems. Apr 25, 1985, Bethesda, MD.
- Koch CG, Figueroa PI, Li L, et al. Red blood cell storage: how long is too long? Ann Thorac Surg 2013;96:1894-9. [Crossref] [PubMed]
- Belizaire RM, Makley AT, Campion EM, et al. Resuscitation with washed aged packed red blood cell units decreases the proinflammatory response in mice after hemorrhage. J Trauma Acute Care Surg 2012;73:S128-33. [Crossref] [PubMed]
- Belizaire RM, Prakash PS, Richter JR, et al. Microparticles from stored red blood cells activate neutrophils and cause lung injury after hemorrhage and resuscitation. J Am Coll Surg 2012;214:648-55; discussion 656-7. [Crossref] [PubMed]
- Chang AL, Kim Y, Seitz AP, et al. Erythrocyte Derived Microparticles Activate Pulmonary Endothelial Cells in a Murine Model of Transfusion. Shock 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Kor DJ, Kashyap R, Weiskopf RB, et al. Fresh red blood cell transfusion and short-term pulmonary, immunologic, and coagulation status: a randomized clinical trial. Am J Respir Crit Care Med 2012;185:842-50. [Crossref] [PubMed]
- Fergusson DA, Hébert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA 2012;308:1443-51. [Crossref] [PubMed]
- Hébert PC, Chin-Yee I, Fergusson D, et al. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg 2005;100:1433-8. table of contents. [Crossref] [PubMed]
- Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med 2015;372:1419-29. [Crossref] [PubMed]
- Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, et al. Effect of Transfusion of Red Blood Cells With Longer vs Shorter Storage Duration on Elevated Blood Lactate Levels in Children With Severe Anemia: The TOTAL Randomized Clinical Trial. JAMA 2015;314:2514-23. [Crossref] [PubMed]
- Dhabangi A, Mworozi E, Lubega IR, et al. The effect of blood storage age on treatment of lactic acidosis by transfusion in children with severe malarial anaemia: a pilot, randomized, controlled trial. Malar J 2013;12:55. [Crossref] [PubMed]
- Weinberg JA, McGwin G Jr, Vandromme MJ, et al. Duration of red cell storage influences mortality after trauma. J Trauma 2010;69:1427-31; discussion 1431-2. [Crossref] [PubMed]
- Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008;358:1229-39. [Crossref] [PubMed]
- Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg 1999;178:570-2. [Crossref] [PubMed]
- Goel R, Johnson DJ, Scott AV, et al. Red blood cells stored 35 days or more are associated with adverse outcomes in high-risk patients. Transfusion 2016;56:1690-8. [Crossref] [PubMed]
- Aubron C, Syres G, Nichol A, et al. A pilot feasibility trial of allocation of freshest available red blood cells versus standard care in critically ill patients. Transfusion 2012;52:1196-202. [Crossref] [PubMed]
- Lacroix J, Hébert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015;372:1410-8. [Crossref] [PubMed]
- Heddle NM, Cook RJ, Arnold DM, et al. The effect of blood storage duration on in-hospital mortality: a randomized controlled pilot feasibility trial. Transfusion 2012;52:1203-12. [Crossref] [PubMed]
- Heddle NM, Cook RJ, Arnold DM, et al. Effect of Short-Term vs. Long-Term Blood Storage on Mortality after Transfusion. N Engl J Med 2016;375:1937-45. [Crossref] [PubMed]


