Malignant pheochromocytoma in the anterior mediastinum with sternal invasion: a case report
Introduction
Primarily arising from neural crest-derived tumors including the adrenal medulla and sympathetic paraganglia, pheochromocytomas consist of chromaffin cells that may produce, store, metabolize and secrete catecholamines (1-3). These tumors commonly originate from approximately 80–85% of cases from intra-adrenal chromaffin tissues and from approximately 15–20% of cases from extra-adrenal chromaffin tissues (4). As the rare disease, more than 40% of pheochromocytomas are associated with germline mutations in susceptibility genes (5). The malignant ones account for approximately 10% of all pheochromocytomas (6,7). Patients who suffer from pheochromocytomas mainly complain of hypertension, headache, sweating, palpitation, apprehension, tremor, pallor or flushing of the face, nausea and vomiting, chest pain, abdominal pain, or limb paresthesias (8,9). These symptoms are regarded as the result of the increased secretion of epinephrine and norepinephrine.
Ectopic pheochromocytomas always originate from the extra-adrenal paraganglion system, which can be either benign or malignant, and may secrete catecholamines. Outside the adrenal glands, pheochromocytomas commonly occur in the organ of Zuckerkandl and paraganglia (10). In addition to the classical symptoms of adrenal pheochromocytomas, the level of urine catecholamine and its derivatives can also be regarded as diagnostic criteria. However, not all extra-adrenal pheochromocytomas secrete these. In fact, some patients who suffer from pheochromocytomas are asymptomatic. To date, the resection of pheochromocytomas remains as the most effective method.
Pheochromocytoma in the anterior mediastinum is very rare, especially those that that invade the sternum (11). Diagnosis and treatment experience is urgently needed due to the proximity of important tissues and organs, including the bone, great vessels and airway. The clinical presentation, diagnostic procedures, and therapeutic strategies are introduced in this rare case to accumulate rich experience.
Case presentation
A 51-year-old female presented with a 2-month history of a mass with pinching pain on the anterior thoracic wall on December 2015. She had undergone resections of the left adrenal giant neoplasm confirmed histologically as pheochromocytoma in 2008 at another hospital (details were unclear). Family history was not definite. No follow-up was performed after discharge from the hospital. She had high blood pressure (BP) for 10 years, and the highest systolic BP reached 230 mmHg. At present, her BP is stable (140/90 mmHg) by daily taking indapamide. On physical examination, a tender and firm 30 mm × 30 mm hard mass was palpated in front of the right third rib cartilage and middle-lower sternum. The patient did not have any headache, dizziness, tachycardia, or dyspnea during the process of the palpation. There was neither burst nor varicose vein on the surface of the mass. Auscultation and percussion on bilateral lungs were normal.
After admission, related auxiliary examination and laboratory tests were performed. The electrocardiogram (ECG) report revealed an incomplete right bundle branch block and ST-T changes. The Holter monitor indicated two accidental atrial premature beats and 591 accidental ventricular premature beats within 24 hours. Additionally, paroxysmal T-wave changes were also found. Chest contrast-enhanced computed tomography (CT) scan revealed a soft tissue mass (approximately 3.7 cm × 4.7 cm × 5.8 cm) on the anterior mediastinum and anterior thoracic wall (Figure 1A,B,C). The mass was likely to be a malignant thymoma. Head CT, abdomen CT and dual-source CT coronary artery imaging revealed nothing abnormal. Heart Doppler ultrasound and pulmonary function test revealed nothing abnormal. Technetium-99 conjugated with methylene diphosphonate (99Tc MDP) bone scintigraphy revealed nuclide focus on the distal mesosternum, first lumbar and fifth lumbar. Meanwhile, for this result, the radiologist could not exclude bone metastases (Figure 1D). Serum cytokeratin 19-fragment diagnostic value was 4.41 ng/mL (normal: 0–3.3 ng/mL). Catecholamine levels including fasting total plasma catecholamines, noradrenaline, urinary noradrenaline and urinary normetanephrine were normal. Additionally, others were within normal ranges including blood routine, serum electrolytes, coagulogram, hepatic function, renal function, thyroid function, serum carcinoembryonic antigen, and squamous carcinoma antigen.
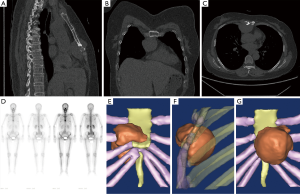
Due to the difficulty of rebuilding low gray tissues, three-dimensional (3D) reconstruction of the tumor, sternum, adjacent costal cartilage and ribs was performed using Mimics 10.01 (Figure 1E,F,G). The video of the reconstructed model revealed the position of the tumor, and helped in the design of the surgical method (Figure 2).

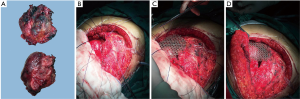
After the completion of diagnostics and interdisciplinary consultations, the patient was qualified for surgical removal of the tumor. When the patient was under general anesthesia, the patient was placed in the supine position. Along the lower edge of the right breast, an anterolateral arc incision (approximately 25 cm) was performed. Then, the incision was lengthened to the mid-mesosternum (approximately 10 cm). Next, the skin and subcutaneous tissue was cut, followed by the upward pushing of the right breast. Then, the third to the seventh rib was exposed before cutting the intercostal muscles and opening the thoracic cavity. The surgeon found the mass located in the middle and lower third of the sternum. It was approximately 5.0 cm × 6.0 cm × 8.5 cm, and only invaded and damaged the sternum (Figure 3A). The tumor adhered closely to the internal thoracic artery, which was possibly nourishing the lesion. There are no planting or metastatic nodules. Then, ligaturing of the right internal thoracic artery, cutting off of the right forth to seventh costal cartilage, transecting the sternum, cutting off of the left fifth to eighth costal cartilage and ligaturing of the left internal thoracic artery were accomplished in sequence. Finally, the mass was completely removed, leaving an irregular circular defect zone (the diameter was approximately 8 cm) (Figure 3B). The return of intraoperative frozen pathology could not except for malignant tumor. After the exact bleeding, a closed thoracic drainage tube was inserted into the right thoracic cavity, and the thoracic cavity was closed. A Medtronic 10 cm ×10 cm titanium mesh was adopted and used to cover the thoracic wall defect with matched screws (Figure 3C,D). Eventually, the incision was sutured layer by layer. Intraoperative anesthetic effect was satisfactory.
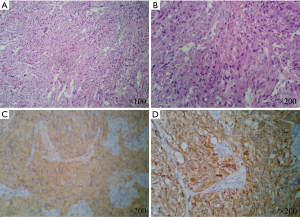
Histological study of the biopsied specimens disclosed pheochromocytomas invading the adjacent bone and skeletal muscle. Microscopically, these tumor cells with atypical nucleolus were round or ovate, possessing an abundant cytoplasm arranged in nests (Figure 4A,B). Immunohistochemistry results demonstrated that neuron-specific enolase (NSE), chromogranin-A (CHGA), α-inhibitin, synaptophysin and S-100 protein were positive; while ki-67 protein was expressed in 15% of these tumors (Figure 4C,D). Combined with clinical history, the final pathological diagnosis was intended to be malignant pheochromocytoma. Her family members refused the suggestion that the surgical specimen could be sent for genetic testing.
On postoperative seventh day, the patient had sudden cough accompanied with white sputum, and could not lie down to sleep. Urgent ECG was performed, which indicated a flat T-wave. Laboratory results revealed that B-type-natriuretic peptide was within the normal range. Therefore, a respiratory physician and cardiologist were invited to consult. Following the consultation notes, oral indapamide was replaced by telmisartan and metoprolol tartrate to better control BP and improve the heart rate.
The patient had an uneventful postoperative recovery without any operative complication such a tracheal compression, tracheomalacia, pneumomediastinum, pneumothorax or cardiovascular blocking. Then, she was discharged and stayed home on the 13th day after the operation in good condition. Since her discharge from the hospital, she has been taking indapamide daily. At 6 months postoperative, the patient was free of symptoms, with no evidence of tumor recurrence.
Discussion
This case presented a rare extra-adrenal pheochromocytoma, which was found within the anterior mediastinum of a 51-year-old female. Mediastinal pheochromocytoma only accounts for 5% of ectopic pheochromocytomas (13). The case in the anterior mediastinum invading the sternum was extremely infrequent. However, this atypical case that appeared in an irregular region may be closely related to its malignancy. Merely one case that described a similar malignancy with sternum destruction has been previously reported (11).
The most common symptoms of pheochromocytomas are headache, blurred vision, sweating, heart palpitation, and flushing (8,9). When pheochromocytomas appear in the mediastinum, patients often complain of palpitation, headache, nausea, vomiting, chest pain, or chest tightness (14-17). However, some of the patients who suffered from pheochromocytomas may not be accompanied by any of these typical symptoms (18). Asymptomatic cases were also found in mediastinal pheochromocytoma patients (19,20). Compared with published cases, this patient suffered from asymptomatic pheochromocytoma (some of the mediastinal pheochromocytoma cases are shown in Table 1).
Therefore, combined with clinical manifestation, radiologic imaging with CT, magnetic resonance imaging (MRI), color Doppler and laboratory tests such as urinary catecholamines and related metabolites could result in an accurate diagnosis. For this case, the painful mass on the anterior thoracic wall was the only clinical manifestation. In fact, we only attained CT and 99Tc MDP results in this case. With the development of radiological techniques, MRI has been regarded as a very reliable examination for detecting other small lesions (21,22). It is also more suitable than CT in the evaluation of pheochromocytomas. Positron emission tomography (PET) has been applied to detect malignant pheochromocytomas with high diagnostic value (15,23). In addition, iodin-131-metaiodobenzylguanidine (I-131-MIBG) scanning, which could detect bone metastasis, was also important for adrenal pheochromocytoma. Compared with 99Tc MDP bone scintigraphy, I-131-MIBG scanning has more diagnostic value for pheochromocytoma. Bone scintigraphy indicated hot spots on the distal mesosternum, first lumbar and fifth lumbar. Furthermore, Bouhouch et al. indicated that distant metastases to the bone, liver, lung, or lymph nodes were the only absolute indicators of malignant pheochromocytoma (19). Therefore, there was one considerable possibility: the tumor in the anterior mediastinum was the primary lesion, while the others were metastases. Similarly, Yamaguchi et al. reported a case about multiple vertebral metastases from malignant cardiac pheochromocytoma (24). Unfortunately, due to family financial difficulties, the patient rejected MRI application. Hence, we also missed the consideration of performing I-131-MIBG scanning. The insufficient information of auxiliary examinations creates difficulties for diagnosis. Therefore, these limited radiographic results only disclosed that the tumor in the anterior mediastinum might be malignant. In fact, the information above was remained inadequate to make a definitive diagnosis.
Pheochromocytoma is commonly treated by a surgical resection. Surgery not only eliminates the lesion, but also takes out the tissue for pathological examination. For better observation and surgical scheme, we accomplished a 3D model of the lesion to fulfill the individualized treatment. Generally, the preoperative and perioperative care of patients with pheochromocytomas should follow several guidelines. Therefore, preparation before an operation is an essential step for functional pheochromocytoma, including a preoperative treatment with α- and β-blocking agents. Complying with the requirement of the patient, we only had an operation on the tumor in the anterior mediastinum, and kept the other two lumbar lesions under followed up. However, due to the uncertain diagnosis of this case, there was shortage of sufficient preoperative and perioperative preparation. When frozen pathology results returned, we made the definite diagnosis of a malignant tumor. Under normal general anesthesia with endotracheal intubation, the lesion was completely resected. Then, the thoracic wall defect was reconstructed with a titanium mesh. Luckily, the surgical progress was smooth without any BP and cardiac problems. Postoperative management was ordinary as usual.
Tatić et al. confirmed that NSE, CHGA and synaptophysin were important pan-neuroendocrine markers in pheochromocytoma diagnosis (25). According to typical pathological characteristic, NSE, CHGA and synaptophysin in this case were all positively expressed. By retrospect of previous medical history, we focused on the patient that underwent the resection operation of a left adrenal giant pheochromocytoma several years ago; although pathology results combined with previous medical history may have determined the diagnosis of metastatic malignant pheochromocytoma. However, WHO defines metastatic malignant pheochromocytoma as the presence of distant metastases in places where chromaffin tissue does not exist (26). Following this definition, the tumor could be thought to originate from the thoracic paraganglia. Thus, for this patient, the diagnosis of primary malignant pheochromocytoma was more appropriate.
After six months follow-up, the patient’s BP was normal and no other symptoms occurred. In addition, suspicious lesions on the first lumbar and fifth lumbar have no development by bone scan. Furthermore, no recurrence of the tumor was found. Moreover, there was still no discomfort on the lumbar.
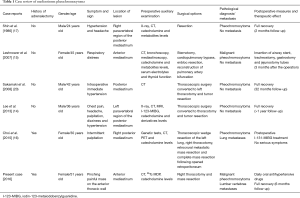
Full table
Conclusions
Malignant pheochromocytoma in the anterior mediastinum invading the sternum is rare and special. A local and painful mass may be the only clinical manifestation without specific radiographic and laboratory results. For this disease, integrated imageological and laboratory examination are necessary. Complete surgical resection of the pheochromocytoma remains as the first choice for these patients. Furthermore, preoperative and post-operation preparations are both crucial. It is worth performing a 3D reconstruction for better observation and individualized treatment. The final decision of the diagnosis should be based on both the pathological results and past medical history. Long-term follow-up is necessary, while other suspicious lesions should also be given sufficient attention.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: This case report is recorded in the Ethics Committee of the First Affiliated Hospital of Dalian Medical University (approval number: LCKY2016-35). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Lenders JW, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet 2005;366:665-75. [Crossref] [PubMed]
- Lenders JW, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA 2002;287:1427-34. [Crossref] [PubMed]
- Eisenhofer G, Lenders JW, Pacak K. Biochemical diagnosis of pheochromocytoma. Front Horm Res 2004;31:76-106. [Crossref] [PubMed]
- Elder EE, Elder G, Larsson C. Pheochromocytoma and functional paraganglioma syndrome: no longer the 10% tumor. J Surg Oncol 2005;89:193-201. [Crossref] [PubMed]
- Fishbein L. Pheochromocytoma and Paraganglioma: Genetics, Diagnosis, and Treatment. Hematol Oncol Clin North Am 2016;30:135-50. [Crossref] [PubMed]
- Strong VE, Kennedy T, Al-Ahmadie H, et al. Prognostic indicators of malignancy in adrenal pheochromocytomas: clinical, histopathologic, and cell cycle/apoptosis gene expression analysis. Surgery 2008;143:759-68. [Crossref] [PubMed]
- Goldfarb DA, Novick AC, Bravo EL, et al. Experience with extra-adrenal pheochromocytoma. J Urol 1989;142:931-6. [PubMed]
- Manger WM. The protean manifestations of pheochromocytoma. Horm Metab Res 2009;41:658-63. [Crossref] [PubMed]
- Jindal V, Baker ML, Aryangat A, et al. Pheochromocytoma: presenting with regular cyclic blood pressure and inverted Takotsubo cardiomyopathy. J Clin Hypertens (Greenwich) 2009;11:81-6. [Crossref] [PubMed]
- Li W, Yang B, Che JP, et al. Diagnosis and treatment of extra-adrenal pheochromocytoma of urinary bladder: case report and literature review. Int J Clin Exp Med 2013;6:832-9. [PubMed]
- Hildebrand J, Haubitz M, Hertel A, et al. Resistant arterial hypertension and a prominent sternum in a 77-year-old woman. Internist (Berl) 2014;55:847-50. [Crossref] [PubMed]
- Song M, Sun K, Xia T, et al. The dynamic display of the reconstructed model of malignant pheochromocytoma. Asvide 2017;4:117. Available online: http://www.asvide.com/articles/1427
- Madani R, Al-Hashmi M, Bliss R, et al. Ectopic pheochromocytoma: does the rule of tens apply? World J Surg 2007;31:849-54. [Crossref] [PubMed]
- Lee JH, Lee SS, Lee JC, et al. Functional mediastinal pheochromocytoma. Korean J Thorac Cardiovasc Surg 2013;46:88-91. [Crossref] [PubMed]
- Leshnower BG, Morris RJ, Pechet TT. Management of an anterior mediastinal pheochromocytoma causing tracheomalacia. Ann Thorac Surg 2007;84:2088-90. [Crossref] [PubMed]
- Choi WS, Park JY, Roh MS, et al. Malignant pheochromocytoma with lung metastasis after right adrenalectomy for pheochromocytoma eleven years ago. J Thorac Dis 2015;7:E37-42. [PubMed]
- Shin MS, Gupta KL, Ho KJ. Thoracic pheochromocytoma: computerized tomographic characteristics. South Med J 1986;79:244-5. [Crossref] [PubMed]
- Bravo EL, Tagle R. Pheochromocytoma: state-of-the-art and future prospects. Endocr Rev 2003;24:539-53. [Crossref] [PubMed]
- Bouhouch A, Hendriks JM, Lauwers PR, et al. Asymptomatic pheochromocytoma in the posterior mediastinum. Acta Chir Belg 2007;107:465-7. [Crossref] [PubMed]
- Sakamaki Y, Yasukawa M, Kido T. Pheochromocytoma of the posterior mediastinum undiagnosed until the onset of intraoperative hypertension. Gen Thorac Cardiovasc Surg 2008;56:509-11. [Crossref] [PubMed]
- Whalen RK, Althausen AF, Daniels GH. Extra-adrenal pheochromocytoma. J Urol 1992;147:1-10. [PubMed]
- Tazi MF, Ahallal Y, Tazi E, et al. Pheochromocytoma of the urinary bladder: a case report. Cases J 2009;2:8585. [Crossref] [PubMed]
- Neumann DR, Basile KE, Bravo EL, et al. Malignant pheochromocytoma of the anterior mediastinum: PET findings with [18F]FDG and 82Rb. J Comput Assist Tomogr 1996;20:312-6. [Crossref] [PubMed]
- Yamaguchi S, Hida K, Nakamura N, et al. Multiple vertebral metastases from malignant cardiac pheochromocytoma--case report. Neurol Med Chir (Tokyo) 2003;43:352-5. [Crossref] [PubMed]
- Tatić S, Havelka M, Paunović I, et al. Pheochromocytoma--pathohistologic and immunohistochemical aspects. Srp Arh Celok Lek 2002;130 Suppl 2:7-13. [PubMed]
- Thompson LD, Young WF, Kawashima A, et al. Malignant adrenal phaeochromocytoma, Benign ohaechromocytoma, Extra-adrenal paragaglioma. In: DeLellis RA, Lloyd RV, Heitz PU, et al. editors. World Health Organisation Classification of Tumors: Pathology and Genetics of Tumors of Endocrine Organs. Lyon: IARC Press 2004:147-55, 159-166.


