Impact of lymph node management on resectable non-small cell lung cancer patients
Introduction
Surgical lung resection with systematic mediastinal lymph node (LN) dissection or sampling is recommended by the National Comprehensive Cancer Network guidelines for stages I–II and resectable stage IIIA non-small cell lung cancer (NSCLC) (1). Previous studies have shown that systematic LN dissection (SLND) could improve staging (2-5) and overall survival compared to LN sampling (6-8). Variations in lung cancer lymphatic drainage may be partly responsible for the need of an extensive mediastinal dissection (9,10). A recent systematic review and meta-analysis showed that SLND was associated with a statistically significant improvement in overall survival and recurrence-free survival (11). However, the effect of the number of dissected LNs on tumor recurrence or overall survival is still controversial. Some studies reported that the number of LNs removed in early stage NSCLC patients who underwent lobectomy was an independent positive prognostic factor for overall and lung overall survival (6,12,13). Other studies suggest that the number of dissected LNs has no significant impact on overall survival, whereas the number of positive LNs does (14,15).
We retrospectively investigated the association between the number of LNs and stations resected, and positivity of dissected LNs to tumor recurrence and overall mortality in clinical early stage NSCLC patients who underwent complete resection at Chiang Mai University Hospital.
Methods
Patient selection
From June 2000 to June 2012, medical records of 240 patients who underwent pulmonary resection for primary lung cancer at the Department of Surgery, Faculty of Medicine, Chiang Mai University Hospital, Chiang Mai, Thailand were retrospectively reviewed. All patients received preoperative cancer staging including computed tomography (CT) with contrast, bronchoscopy with biopsy, bronchial washing, brushing or bronchial lavage cytology. If mediastinal lymph nodes were larger than 1 cm, endobronchial ultrasound-guided (EBUS) fine needle aspiration, or mediastinoscope biopsy were performed. Patients with mediastinal lymph nodes involvement were classified as N2 disease, and induction chemotherapy was performed. Positron emission tomography-computed tomography (PET-CT) was not available. CT-brain and bone scans were performed if clinically relevant. These patients represent all clinical early stage NSCLC who underwent curative intent-complete pulmonary resection with SLND or sampling of the hilum and mediastinum according to the international LN map for TNM classification of lung cancer (16). Exclusion criteria were patients who received any induction therapy (radiation or chemotherapy). NSCLC staging was determined according to the 7th edition of the TNM classification of malignant tumors (17). The World Health Organization classification, 3rd edition was used for histological tumor types. All dissected LNs were examined pathologically by expert pulmonary pathologists (Nirush Lertprasertsuke, Sarawut Kongkarnka) who were blinded to the clinical outcomes. To confirm the clinical early status, all patients went through a preoperative evaluation, including medical history, physical examination, plain chest radiography, chest CT, and bone scan or PET-CT if necessary.
Surgical procedures included sublobar resection [3 patients (1.3%) with clinical stage Ia and age 74, 75 and 78 years], lobectomy (232 patients, 96.7%) and pneumonectomy (5 patients, 2.1%) depending on tumor size and location. Mediastinal LN dissection was routinely performed; in some patients, for example T1 tumor in patients age >70 years, A LN sampling was done. LN stations were defined according to the IASLC classification (18). The N2 level included ipsilateral station 2–station 9, N1 level included ipsilateral station 10–station 14, N3 level included station 1, contralateral mediastinal LN, and hilar nodes. Mediastinal LN dissection or sampling was routinely performed after complete pulmonary resection. All dissected LNs were collected in separate labeled-containers according to the dissected stations. Tissues and LNs were sent for routine pathologic analysis and preserved in paraffin blocks. All patients were followed and evaluated for clinical symptoms and a chest X-ray at the thoracic surgery clinic at 2 weeks and at 3 months after surgery; then every 6 months for CT of the chest within the first 2 years after surgery, and then every year after that.
The primary outcomes of this study were tumor recurrence and overall mortality.
Tumor recurrence was defined as the first diagnosis of either local recurrence or distant metastasis after complete surgical resection. This study was reviewed and approved by the Institutional Review Board of Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand with Study Code: SUR-2556-01811/Research ID: 1811, and ID of the approval 276/2013.
Statistical analysis
Categorical variables were presented as frequencies and percentages. Continuous variables were presented as means and standard deviation (SD) or median and interquartile range (IQR) as appropriate, and compared by the Student t-test or Wilcoxon Rank Sum test. The impact of the number of LNs resected and positivity of dissected LNs on tumor recurrence and overall death was performed using multilevel parametric survival models adjusting for age, gender, comorbidities, surgical procedures, pathologic findings (histologic cell types, tumor grading, intratumoral lymphatic invasion, intratumoral vessel invasion, visceral pleural invasion, tumor necrosis, and stage of disease), and type of LN dissection (analyzed under stratification of identical number of dissected LNs or positivity of dissected LNs). Dependent variables were number of dissected LNs and positivity of dissected LNs. The positivity of dissected LNs was calculated as the number of tumor positive LNs divided by total number of dissected LNs multiplied by 100. Time to recurrence was calculated from the date of surgery to the time of a diagnosis of tumor recurrence. The overall death was calculated from the time of surgery to the time of death. Recurrent-free parametric survival curves according to the optimal cut-point number of dissected LNs overall and for each NSCLC stage was analyzed by mixed-effects Weibull regression. All tests were two sided. A P value less than 0.05 was considered statistically significant. STATA program version 14.0 (StataCorp, CS, TX, USA) was used for statistical analysis.
Results
Patient characteristics, treatments and pathologic findings
Most of the patients included in the study were ex-smokers (Table 1); male to female ratio was 1.4:1. The most common presenting symptoms were chronic cough and non-massive hemoptysis. Asymptomatic patients were approximately 37%. Lung mass or nodule was found by incidental finding from chest X-ray.
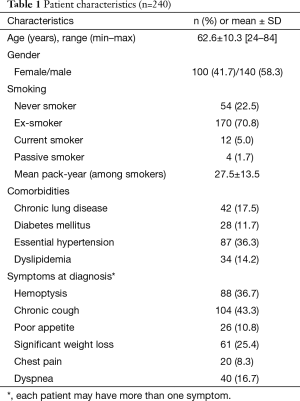
Full table
The surgical procedures and pathologic findings were shown in Table 2. Lobectomy was performed in most cases (96.7%). Sublobar resection included wedge resection (1 patient) and segmentectomy (2 patients). Pneumonectomy was performed in five cases due to intraoperative findings of progressive hilar tumor. The two most common histologic types were adenocarcinoma (61.3%) and squamous cell carcinoma (27.5%). Pathological stages were 33.8% for stage I, 49.1% for stage II, and 32.9% for stage III. SLND was performed in most cases (87.1%). The mean number of dissected LNs was 4.3 nodes for sampling and 25.5 nodes for SLND. In most cases (73.8%), LN dissection was performed in more than 5 stations. The mean of positivity of dissected LNs was 10.4±18.4. There was no difference in number of dissected LNs between right and left side [23.1±12.6 and 22.0±13.2 (P value 0.519) respectively]. Tumor recurrence occurred in 51.3% of patients, with median (IQR) recurrent time of 9.5 (6.0–17.9) months. Overall death occurred in 43.3% of patients, with median (IQR) follow-up time of 23.9 (12.5–47.6) months.
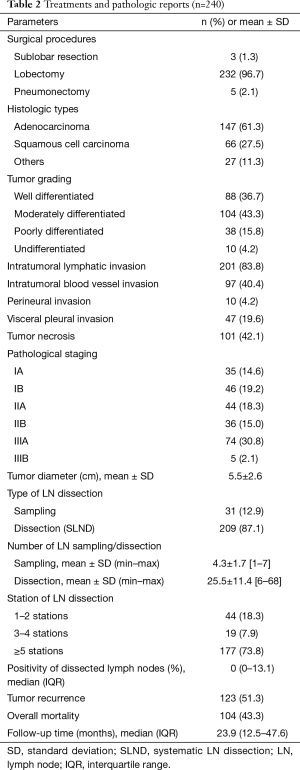
Full table
Association between LN resection and tumor recurrence
The number and positivity of dissected LNs were statistically significant prognostic factors for tumor recurrence (Table 3) at univariable analysis [hazard ratios (HR) and 95% confidence interval (CI) of 0.98 (0.97–0.99) and 1.01 (1.01–1.02), respectively]. At multivariable analysis, the number and positivity of dissected LNs were independent prognostic factors for tumor recurrence (Table 4). When the analysis was stratified according to the stage of disease, the association between number of dissected LNs and tumor recurrence was restricted to stage I. There was an inverse correlation between positivity of dissected LNs and recurrent rate and overall mortality (Figure 1).
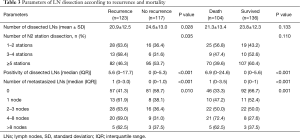
Full table
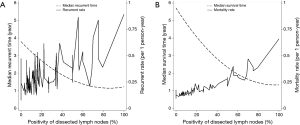
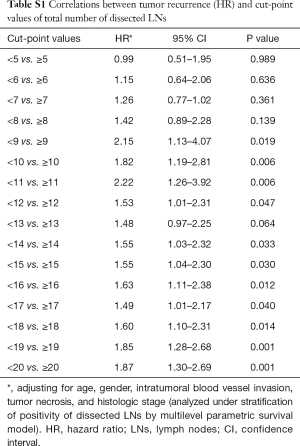
The optimal cut-off point for total number of dissected LNs was 11 (HR for recurrence 2.22, 95% CI: 1.26–3.92; Table S1). The strongest association was observed in stage I and stage II NSCLC as shown by adjusted parametric survival curve analyzed by mixed-effects Weibull regression (Figure 2). The unadjusted Kaplan-Meier survival curves and the number of at risk patient were shown in Figure S1.
Full table
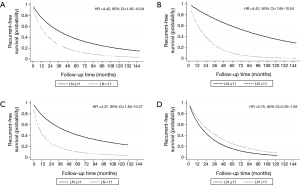
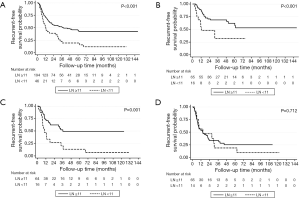
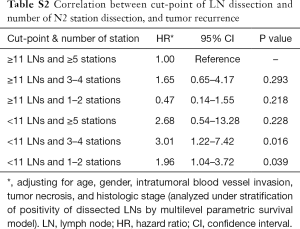
The location of the dissected LNs was not an independent prognostic factor for tumor recurrence (Table 4). However, if less than 11 LNs were resected, with ≤5 stations dissected, a significant worse recurrence prognosis was observed (Table S2, the interested reader can find a supplementary appendix in online).
Full table
Full table
The number of metastasized LNs was not an independent prognostic factor for tumor recurrence at multivariable analysis (Table 4).
Association between LN resection and overall mortality
The association between number of dissected LNs, the number of N2 stations, the positivity of dissected LNs, and the number of metastasized LNs and overall mortality is shown in Table 3. The total number and locations (stations) of dissected LNs were not an independent prognostic factor for overall mortality (HR 0.99, 95% CI: 0.97–1.01), whereas the positivity of dissected LNs was (HR 1.02, 95% CI: 1.01–1.02) for each increasing percentage of positive LN. At multivariable analysis, the positivity of dissected LNs was an independent prognostic factor for overall mortality, whereas the total number and number of stations of dissected LNs did not affect overall mortality (Table 4). There was an inverse correlation between positivity of dissected LNs and overall mortality (Figure 1B). The number of metastasized LNs was significantly different with survival status at univariable analysis (Table 3), but not at multivariable analysis (Table 4).
Discussion
LN status is a major prognostic factor for tumor recurrence and survival in lung cancer patients. However, mediastinal LN dissection as part of NSCLC treatment is still controversial. The present results indicate that tumor recurrence and overall mortality after complete pulmonary resection and LN dissection are associated with the presence of positive LNs among those dissected. However, the number of LNs dissected is associated with tumor recurrence, but not with overall mortality. The cut-off point for recurrence was 11 LN in this study. Patients having had less than 11 LNs dissected had significantly less recurrent-free survival, but no effect on overall mortality. Previous studies have shown different results, with number of LNs dissected affecting both overall survival and lung overall survival (2,6,7,12). Possible reasons for the discrepancy could be the small sample size of this present study which carries less statistical power, the shorter follow-up time, or different techniques of data analysis. Some previous studies reported on the association between recurrence and number of dissected LNs (13,19). In this study the number of dissected LNs was an independent prognostic factor for tumor recurrence. Previous studies found that the optimal cut-point numbers of dissected LNs was varied, in the range of more than 6 to 15 LNs (2,13,19-21). In the present study, 10 or more LNs should be dissected in order to obtain a significant decrease in tumor recurrence, and this is especially true in pathologic stage I and II NSCLC.
The stations dissected are an important factor when performing LN dissection. Currently, the number of LN stations to be dissected is still under debate. Wang et al. suggested dissection of only 3 stations of N2 (7). Gajra et al. found that patients with a larger number of mediastinal stations dissected had an improvement in disease-free and overall survival compared to those with fewer mediastinal stations dissected, and suggested a dissection of more than 4 stations (13), similar to what was performed in this study. However, in the present study the number of N2 stations dissected was not an independent prognostic factor for tumor recurrence and overall mortality. The dissection of less than 5 stations and less than 11 LNs suggested significant adverse outcomes in terms of tumor recurrence. Recently, Liang et al. reported data from a Chinese multi-institutional registry and the US SEER database on stage I to IIIA resected NSCLC [2001–2008]; in this paper, a larger number of examined LNs was associated with more-accurate node staging and better long-term survival. The authors recommended 16 LNs as the cut point for evaluating the quality of LN examination and declaring a node-negative disease (22). However, the data of the stations of dissected LNs was not available in that publication.
Nwogu et al. used the SEER database to explore the prognostic value of the number of LNs examined and the ratio of metastatic LNs to total number of dissected LNs (positivity); and found that the more LNs are resected and the lower the ratio of positive LNs to total examined LNs, indicated better survival (23). The present study also indicated that the positivity of dissected LNs was a significant prognostic factor for tumor recurrence and overall mortality. Therefore, the number of metastatic LNs may be more important than the total number of dissected LNs. Previous studies reported that the number of metastatic LNs can predict the outcome after NSCLC complete resection, and that this is a strong independent prognostic factor (15,24,25). It was suggested that this approach provides more accurate pathologic nodal staging then the method of considering the anatomical location of involved LNs (26). Saji et al. found that four or more affected LNs were a good indicator of outcome after surgery (25). Jonnalagadda et al. also reported that the number of affected LNs was an independent prognostic factor for overall survival in patients with N1 NSCLC (15). However, all previous studies analyzed the data with the Cox proportional hazards model, with adjustment for other potential prognostic factors. In this study, we analyzed both the positivity of dissected LNs and the total number of dissected nodes using a multilevel parametric survival model under the assumption of an identical number of dissected LNs and adjusting for other potential prognostic factors. Since there was statistical evidence that both the number of total LNs dissected and LN positivity were associated with tumor recurrence and mortality, it is important to quantify the effect of the number of LNs dissected on the two outcomes conditional on LNs positivity, and vice-versa. This analysis was performed under the multilevel (or conditional) parametric model which is statistically more efficient than the traditional Cox’s model given the relatively small sample size.
This specific statistical analysis is one of the strengths of this study. There are some limitations in this study because of its retrospective nature. First, clinical stage was not available in the database; therefore it was not possible to compare stage before and after surgery (up-stage). However, all patients in this cohort were clinically resectable, thus the maximum nodal diameter of mediastinal LNs at CT scan was less than 1 cm, and were classified as clinical N0 patients. Second, the small sample size of this study may carry a lower statistical power than previous studies. Further large cohort studies, more complete data collection, and/or multi-center studies are warranted. Third, in 13% of patients LN sampling was performed, following ACCP guidelines (3) that recommend either SLND or sampling in early clinical stage, especially in stage I disease. SLND was routinely performed in all cases; in patients age >70 years with small tumor size (less than 1 cm) in the upper lobe, we performed sublobar resection or lobectomy with LN sampling. In order to address this possible bias which may be associated with patient survival or tumor recurrence, we adjusted the multivariable analyses for type of LN dissection (among other covariates. Finally, some important pre-treatment clinical features including the Charlson comorbidity index, pulmonary function test or other cardiopulmonary test, and patient’s socioeconomic status that may be associated with overall mortality are not available for adjusted in multivariable model.
Conclusions
This present study indicates that a dissection of less than 11 LNs, with less than 5 LN stations harvested is inversely associated with tumor recurrence after complete resection in clinical early stage NSCLC patients. Moreover, the positivity of dissected LN is an independent prognostic factor for tumor recurrence and overall mortality. SLND should be performed not only for identifying the exact positivity of dissected LN, but also for determining accurate nodal staging for NSCLC.
Acknowledgements
The authors would like to thank Miss Erlin Daley, research administrator of the Department of Thoracic Surgery, Icahn Medical School at Mount Sinai for review the English manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was designed as retrospective and approved by the Institutional Review Board of Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand with study code: SUR-2556-01811/Research ID: 1811, and ID of the approval 276/2013. Because this study is retrospective in nature and all procedures were routinely performed as part of the patients’ standard of care, the participants did not sign a specific research informed consent. However, all patients released their fully-informed written consent to perform all surgical procedures.
References
- Schapira MM, Aggarwal C, Akers S, et al. How Patients View Lung Cancer Screening. The Role of Uncertainty in Medical Decision Making. Ann Am Thorac Soc 2016;13:1969-76. [Crossref] [PubMed]
- Zhou H, Tapias LF, Gaissert HA, et al. Lymph Node Assessment and Impact on Survival in Video-Assisted Thoracoscopic Lobectomy or Segmentectomy. Ann Thorac Surg 2015;100:910-6. [Crossref] [PubMed]
- Scott WJ, Howington J, Feigenberg S, et al. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:234S-242S.
- Wu N, Yan S, Lv C, et al. Comparison of systematic mediastinal lymph node dissection versus systematic sampling for lung cancer staging and completeness of surgery. J Surg Res 2011;171:e169-73. [Crossref] [PubMed]
- Keller SM, Adak S, Wagner H, et al. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg 2000;70:358-65; discussion 365-6. [Crossref] [PubMed]
- Ou SH, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol 2008;3:880-6. [Crossref] [PubMed]
- Wang X, Yan S, Phan K, et al. Mediastinal lymphadenectomy fulfilling NCCN criteria may improve the outcome of clinical N0-1 and pathological N2 non-small cell lung cancer. J Thorac Dis 2016;8:342-9. [Crossref] [PubMed]
- Wu Yl, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. [Crossref] [PubMed]
- Ndiaye A, Di-Marino V, Ba PS, et al. Anatomical variations in lymphatic drainage of the right lung: applications in lung cancer surgery. Surg Radiol Anat 2016;38:1143-51. [Crossref] [PubMed]
- Topol M, Masłoń A. The problem of direct lymph drainage of the bronchopulmonary segments into the mediastinal and hilar lymph nodes. Clin Anat 2009;22:509-16. [Crossref] [PubMed]
- Meng D, Zhou Z, Wang Y, et al. Lymphadenectomy for clinical early-stage non-small-cell lung cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2016;50:597-604. [Crossref] [PubMed]
- Ludwig MS, Goodman M, Miller DL, et al. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 2005;128:1545-50. [Crossref] [PubMed]
- Gajra A, Newman N, Gamble GP, et al. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol 2003;21:1029-34. [Crossref] [PubMed]
- Riquet M, Legras A, Mordant P, et al. Number of mediastinal lymph nodes in non-small cell lung cancer: a Gaussian curve, not a prognostic factor. Ann Thorac Surg 2014;98:224-31. [Crossref] [PubMed]
- Jonnalagadda S, Smith C, Mhango G, et al. The number of lymph node metastases as a prognostic factor in patients with N1 non-small cell lung cancer. Chest 2011;140:433-40. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Vallières E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049-59. [Crossref] [PubMed]
- Bria E, Milella M, Sperduti I, et al. A novel clinical prognostic score incorporating the number of resected lymph-nodes to predict recurrence and survival in non-small-cell lung cancer. Lung Cancer 2009;66:365-71. [Crossref] [PubMed]
- Yang M, Cao H, Guo X, et al. The number of resected lymph nodes (nLNs) combined with tumor size as a prognostic factor in patients with pathologic N0 and Nx non-small cell lung cancer. PLoS One 2013;8:e73220. [Crossref] [PubMed]
- Sawyer TE, Bonner JA, Gould PM, et al. Patients with stage I non-small cell lung carcinoma at postoperative risk for local recurrence, distant metastasis, and death: implications related to the design of clinical trials. Int J Radiat Oncol Biol Phys 1999;45:315-21. [Crossref] [PubMed]
- Liang W, He J, Shen Y, et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol 2016.JCO2016675140. [Epub ahead of print]. [PubMed]
- Nwogu CE, Groman A, Fahey D, et al. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann Thorac Surg 2012;93:1614-9; discussion 1619-20. [Crossref] [PubMed]
- Lee JG, Lee CY, Park IK, et al. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg 2008;85:211-5. [Crossref] [PubMed]
- Saji H, Tsuboi M, Yoshida K, et al. Prognostic impact of number of resected and involved lymph nodes at complete resection on survival in non-small cell lung cancer. J Thorac Oncol 2011;6:1865-71. [Crossref] [PubMed]
- Wei S, Asamura H, Kawachi R, et al. Which is the better prognostic factor for resected non-small cell lung cancer: the number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol 2011;6:310-8. [Crossref] [PubMed]


