Phenotypic presentation of chronic cough in children
Introduction
Coughing is a complex respiratory act. During this neuromuscular phenomenon, various respiratory and extra-respiratory muscles are involved, and multiple peripheral and central neural circuits of the cough reflex are activated (1,2). Understanding the mechanisms behind a cough is helpful for effective management. The last 10 years have witnessed a growing interest in basic and clinical research on cough. Drastic changes in our knowledge of the neurophysiology of cough and novel clinical entities have recently emerged (1-5). Moreover, studies have revealed the pathophysiological differences between children and adults with chronic cough (6).
Chronic cough in children is increasingly defined as cough that lasts more than 4 weeks rather than the universally defined 8 weeks in adults (7). Post-infectious cough, asthma, bronchiectasis, malacia, and protracted bacterial bronchitis (PBB) appear to the major causes of cough in young children. By adolescence, the causes of cough are more likely to be similar to those in adults, namely, gastroesophageal reflux, asthma, and upper airway syndrome (8). These differences are attributed to various characteristics of children (Table 1).
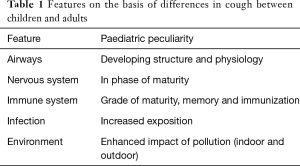
Full table
Although the concept of individualized diagnosis and treatment of chronic cough is attractive, it is highly debated how to pursue it in a clinical setting. For this purpose, a thorough clinical profile describing the child may be helpful in identifying management strategies.
A correct interpretation of the phenotypic presentation can be translated into guidance for workup. Regardless of the setting and age, children with chronic cough should be evaluated carefully using protocols specific to children (7). Knowledge of the pathophysiology of the various conditions that cause chronic cough is vital to its correct diagnosis and management. The use of cough management protocols or algorithms improves clinical outcomes and cough management. Algorithms should differ depending on the associated characteristics of the cough and clinical history.
Wet and dry cough are major phenotypic traits of chronic cough in children (9). Both are potential indicators of pathological conditions, although a transformation from one condition to the other can occur.
The vibration of larger airways and laryngeal structures causes the cough sound during a turbulent flow in expiration. Mucus in the large airways, as opposed to the small airways, is required for a detectable difference in cough quality. The rheological properties of mucus influence cough sounds, and the shearing of the secretions from the airways contributes to the sounds. Numerous investigations have underlined the helpfulness of the cough’s sound (dry or wet) in guiding the diagnostic approach (9,10). In addition to their ability to describe the cough sound, parents currently record cough outbreaks using their smart phones.
Although a plethora of diagnostic options exist, initial testing should rely heavily on guidance from the history and physical examination. Ruling out the signs and symptoms associated with red flags is essential in this initial phase. Performing a thorough history and physical examination is crucial to starting an individualised workup. It is essential to collect history in a setting with empathy for the family and the child.
In this article, we present our knowledge of the phenotypic presentations of the major emerging causes of chronic cough in children.
PBB
Although PBB has been a known pathological condition in children, it has been listed under different names, including persistent endobronchial infection, chronic bronchitis or pre-bronchiectasis. Comprehensive studies of PBB were lacking until 2016, when researchers from Australia focused research on this disease (11-13). These investigations have characterized the clinical profile (Table 2), immunological alterations and structural anomalies of the airways in PBB.

Full table
The original “microbiologic-based” definition (coined in 2006) characterized PBB as follows (11):
- Presence of a chronic wet cough (>4 weeks);/
- Lower airway infection (recognized respiratory bacterial pathogens growing in the sputum or at BAL at density of a single bacterial specifies >104 colony-forming units/mL);
- Cough resolves following a 2-week course of an appropriate oral antibiotic (usually amoxicillin-clavulanate).
Studies of airway microbiology in PBB have revealed lower airway bacterial infection with H. influenzae, M. catarrhalis and/or S. pneumonia (11,14,15). Moreover, children with PBB had significantly elevated percentages of neutrophils in the lower airways compared with control subjects, and adenovirus was more likely to be detected in BAL specimens of those with PBB (OR, 6.69; 95% CI: 1.50–29.80) (16).
Preschool children are unable to expectorate, and studies of their lower airways rely on bronchoscopic assessment to provide definitive evidence for lower airway bacterial infection in PBB. In addition to being an invasive procedure, bronchoscopy is not adaptable in primary care settings.
PBB was recently clinically defined as a condition characterized as follows (5):
- Presence of a continuous chronic (>4-week duration) wet or productive cough;
- Absence of symptoms or signs (i.e., specific cough pointers) suggestive of other causes of wet or productive cough;
- Cough resolves following a 2- to 4-week course of an appropriate oral antibiotic.
Although children with PBB have wet cough, they usually lack systemic symptoms. The majority are preschool-aged and are likely to have attended childcare facilities [odds ratio (OR) =8.4, 95% confidence interval (CI): 2.3–30.5] (16). They typically appear well and lack signs of an underlying disease other than wet cough, although a ‘rattly chest’ and crackles are occasionally heard in these children. A chest radiograph is usually normal or only shows peri-bronchial changes. Unfortunately, for children attending childcare facilities, the children and their families may learn to live with this condition for months. In other cases, PBB may be misdiagnosed as asthma (night time cough) (11,14). PPB is frequently associated with malacia (11,12,14).
Awareness of PBB is currently increasing based on clinical observations showing that recurrence is a potential precursor for chronic suppurative lung disease or bronchiectasis.
In a recent study, Wurzel et al. investigated risk factors for developing bronchiectasis in children with PBB. Major risk factors for bronchiectasis included lower airway infection with H. influenzae (recovered in BAL fluid) (P=0.013) and recurrent episodes of PBB (P=0.003) (17). H. influenzae infection conferred more than a seven-fold higher risk of bronchiectasis (hazard ratio, 7.55; 95% CI: 1.66–34.28; P=0.009) compared with no H. influenzae infection.
Bronchiectasis
Non-cystic fibrosis bronchiectasis is a structural abnormality of the airways characterized by abnormal dilation and distortion, leading to recurrent chronic airway infections and inflammation (18). This condition is caused by a variety of pathophysiologic processes. Multiple genetic, anatomical, and systemic causes of bronchiectasis have been identified. Regardless of the underlying cause of bronchiectasis, all patients share a common denominator of mucus retention and superimposed bacterial colonization. The mechanisms behind the lung damage seem quite similar. Protracted, persistent or recurrent infections and amplified neutrophilic inflammation are the basis of lung injury (18,19). These injuries are typically associated with impaired mucociliary clearance and poorly regulated inflammatory responses.
In the last few years, bronchiectasis in children and adults is attracting major clinical and research interest (19,20). Determining the cause of the disease is a key priority for the physician. An appreciation of the aethiopathogenesis of bronchiectasis is crucial in the management of this disease.
The causes of bronchiectasis in childhood are various. What should be kept in mind is that bronchiectasis is not a rare finding in this age. Moreover, children have various anatomical and structural factors that predispose the airways to damage. In Childhood, structural changes in the growing lungs include alveolar growth and multiplication, growth and maturation of the lung parenchyma, vascular development, growth of the airways and maturation of the airway wall structures, all of which are influenced by the simultaneous growth of the thoracic cage (21-23). Infections, immunodeficiencies, recurrent aspiration and primary ciliary dyskinesia account for most cases of bronchiectasis.
In paediatrics, a diagnosis of bronchiectasis is generally established on the basis of clinical symptoms (Table 3) and signs in addition to distinctive radiological findings using a smaller broncho-arterial ratio of >0.8 (for adults, the ratio is 1) (24). However, the reversibility of bronchiectasis on imaging has been shown in several studies in children with cylindrical bronchiectasis.
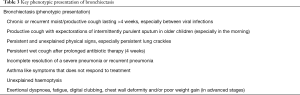
Full table
Clinically, children with bronchiectasis present with a continuous wet cough or recurrent episodes of wet cough and recurrent chest infections. Older children may show a productive cough with expectorations of intermittently purulent sputum. Symptoms may be aggravated with physical activity, leading to limited exercise tolerance. Crackles may be found on auscultation, but their absence does not exclude bronchiectasis. Clubbing and haemoptysis are rarely present. Recently, Goyal et al. proposed that a computerised tomography scan should be considered in a child with a chronic wet cough that persists following 4 weeks of oral antibiotics (25).
The diagnosis should be suspected in any child with chronic or recurrent wet cough that is not responding to antibiotics, especially if the cough is accompanied by exertional dyspnoea, recurrent wheezing and chest infections, haemoptysis, digital clubbing or chest wall deformity (26,27).
King et al. investigated 182 subjects with bronchiectasis and distinguished between two phenotypes of patients with bronchiectasis: those who had developed a chronic productive cough in childhood (before 16 years of age) and those who had developed a productive cough as adults (28). The clinical features of the childhood onset and adult onset groups differed for several factors. Moreover, the authors observed that there was also a bi-modal distribution of the age of onset of productive cough, with the most common age of onset in the first 15 years of life followed by the onset of productive cough in subjects over the age of 50. These findings suggest that immune function is best between these age groups. It has been well established that immune function declines with age starting from approximately 50 years old (29).
More interesting is the finding by Field, who observed that as children became adults, their symptoms improved regardless of treatment. Thus, prevention of bronchiectasis in children is highly recommended.
Malacia
Malacia is a condition characterized by abnormal airway collapsibility caused by weakness of the airway walls and its supporting cartilage as well as by the increased flaccidity of the membranous portion of the central airways (30). This disorder may arise congenitally (from disorders associated with impaired cartilage maturation or in combination with other anomalies, such as tracheoesophageal fistula or congenital syndromes) or may be acquired. The latter includes intubation, trauma, infection, chronic inflammation or long-standing extrinsic compression by components of tumour, vascular, skeletal, or cystic origins (31). Malacia is a neglected pathological condition; however, the finding that it is responsible for considerable airway morbidity is generating interest. Malacia may affect one or both bronchi and/or the trachea. Tracheomalacia results in soft tracheal walls that collapse during respiration. The collapse causes airflow obstruction and wheezing, stridor, or both (32,33). If the lesion is extrathoracic, the collapse and airway sounds occur primarily during inspiration. If the lesion is intrathoracic, the collapse and airway sounds occur primarily during exhalation. Bronchomalacia is caused by deficiency in the bronchial cartilage and is defined as an appearance of deformity and a bronchial cross-sectional decrease greater than or equal to 50% during exhalation. The prevalence of tracheobronchomalacia in children is unknown because data come from studies performed on selected populations rather than the general population.
Tracheobronchial malacia symptoms are nonspecific and overlap with other pulmonary disorders; thus, clinical diagnosis is difficult. In many cases, parents are able to recognize their child just by hearing his cough. The cough is characterised by a regular biphasic pulsating sound, seal-like bark, presumably the result of expiratory collapse and vibration of the floppy membranous wall against the anterior airway wall. During cough, the cross-sectional area of the trachea may be reduced up to 80%.
The instability of the tracheal wall is such that the more marked, dynamic compression of the trachea that ensues during coughing may impair rather than enhance the cough’s efficiency in clearing mucus, producing the characteristic brassy sound of the cough (32).
Chang et al. showed that an unusual quality of cough, such as a vibratory or brassy cough, has high levels of association with bronchoscopically proven malacia (10). In addition, several studies have demonstrated that a high percentage of children with chronic cough also have TBM. It is still questionable whether children are more prone to malacia than adults due to their structural immaturity.
This diagnosis should be considered in children who have persistent or protracted recurrent airway symptoms, such as persistent or recurrent “wet” cough, unusual cough, expiratory stridor, wheeze, rattly respiration or dyspnoea/respiratory distress (Table 4) (20,31,33,34). Suspicion of tracheobronchial malacia should arise in children who have syndromes involving cardiac disorders, tracheoesophageal fistula, bronchopulmonary dysplasia, and happy wheezers and all those who have undergone prolonged intubation or tracheotomy (31).
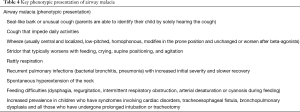
Full table
In school children, spirometric evaluation is helpful. Abnormalities in flow-volume loops may suggest airway collapse, such as compression (35).
Many diagnostic tools are currently employed in the workup, such as computed tomography, dynamic magnetic resonance imaging and fibre-optic or virtual bronchoscopy (36).
Post-infectious cough
Respiratory viruses (particularly respiratory syncytial virus, influenza, parainfluenza and adenovirus), Mycoplasma pneumoniae, Chlamydia pneumoniae and Bordetella pertussis are the main agents that may induce prolonged cough for more than 4 weeks (37). Initially, post-infectious chronic cough was attributed to the extensive disruption of epithelial integrity and airway-induced inflammatory responses in the upper and/or lower airways, which were suspected to expose sensory nerve endings in the airway lining to prolonged and excessive stimulation (38). However, recent experimental studies have demonstrated that post-infectious cough is a complex process that involves cough receptors in the afferent nerves and peripheral and central neural circuits involved in the cough reflex (39,40). Post-infectious cough is self-limited, usually resolves in time, and appears to be the tail end of an infection. Pertussis infection remains the most common cause of post-infectious chronic cough. Cough may persist for months after acute infection.
Wang et al. demonstrated that the cough duration in children with positive Mycoplasma pneumoniae serology (median: 39 days, 95% CI: 24–54) was significantly shorter than in children with positive pertussis serology (median: 118 days, 95% CI: 82–154, P<0.001) (41). Mycoplasma pneumoniae and/or Chlamydia pneumoniae play a significant role in community-acquired LRTIs in children of all ages.
In many respiratory infections, cough is often the last symptom to disappear. One-third of children who initially have a treated empyema are still coughing by 4 weeks, one-quarter are still coughing at 6 months, and approximately 3% are coughing at 12 months (42).
A gradually waning cough is typical of post-infectious cough. A careful history and inquiry on the timing of coughing outbreaks are helpful in differential diagnosis. Diagnostic workup may include laboratory testing, chest X-ray or spirometry to confirm diagnosis.
In a recent study, Chang et al. showed that children with spontaneously resolving cough are older children with dry cough and no wheeze, but these children had a normal chest X-ray and spirometry (9).
Other causes of chronic cough, such as asthma, gastroesophageal reflux, inhalation syndromes, upper respiratory syndrome, and somatic or habit cough, should follow specific guidelines for diagnosis and treatment.
Conclusions
Chronic cough in children may be representative of a simple, spontaneously resolving cough or a specific, serious disorder. Children with chronic cough should be evaluated carefully using protocols specific to children. Paediatric guidelines and clinical algorithms have identified pointers or “red flags” to consider during investigations. A correct interpretation of the phenotypic presentation can be translated into guidance for workup in primary care. Performing a thorough history and physical examination is crucial to starting an individualised workup. Preventative strategies for chronic cough in children include reduced exposure to infectious agents, the use of adequate antibiotics, immunization and easy access to health systems. Care of children with chronic cough should be conducted by skilled paediatric centres. Future clinical and research challenges include understanding the various processes of chronic cough and improving its management in childhood.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Canning BJ, Chang AB, Bolser DC, et al. Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest 2014;146:1633-48. [Crossref] [PubMed]
- Mazzone SB, Undem BJ. Vagal afferent innervation of the airways in health and disease. Physiol Rev 2016;96:975-1024. [Crossref] [PubMed]
- Morice AH, Millqvist E, Belvisi MG, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J 2014;44:1132-48. [Crossref] [PubMed]
- Smith JA, Woodcock A. Chronic cough. N Engl J Med 2016;375:1544-51. [Crossref] [PubMed]
- Chang AB, Upham JW, Masters IB, et al. Protracted bacterial bronchitis: The last decade and the road ahead. Pediatr Pulmonol 2016;51:225-42. [Crossref] [PubMed]
- Chang AB, Robertson CF, Van Asperen PP, et al. A multicenter study on chronic cough in children: burden and etiologies based on a standardized management pathway. Chest 2012;142:943-50. [Crossref] [PubMed]
- Chang AB, Oppenheimer JJ, Weinberger MM, et al. Use of management pathways or algorithms in children with chronic cough: CHEST Guideline and Expert Panel Report. Chest 2017. [Epub ahead of print].
- Kantar A, Bernardini R, Paravati F, et al. Chronic cough in preschool children. Early Hum Dev 2013;89 Suppl 3:S19-24. [Crossref] [PubMed]
- Chang AB, Van Asperen PP, Glasgow N, et al. Children with chronic cough: when is watchful waiting appropriate? development of likelihood ratios for assessing children with chronic cough. Chest 2015;147:745-53. [Crossref] [PubMed]
- Chang AB, Gaffney JT, Eastburn MM, et al. Cough quality in children: a comparison of subjective vs. bronchoscopic findings. Respir Res 2005;6:3. [Crossref] [PubMed]
- Marchant JM, Masters IB, Taylor SM, et al. Evaluation and outcome of young children with chronic cough. Chest 2006;129:1132-41. [Crossref] [PubMed]
- Wurzel DF, Marchant JM, Yerkovich ST, et al. Prospective characterization of protracted bacterial bronchitis in children. Chest 2014;145:1271-8. [Crossref] [PubMed]
- Marchant JM, Gibson PG, Grissell TV, et al. Prospective assessment of protracted bacterial bronchitis: airway inflammation and innate immune activation. Pediatr Pulmonol 2008;43:1092-99. [Crossref] [PubMed]
- Donnelly D, Critchlow A, Everard ML. Outcomes in children treated for persistent bacterial bronchitis. Thorax 2007;62:80-4. [Crossref] [PubMed]
- Kompare M, Weinberger M. Protracted bacterial bronchitis in young children: Association with airway malacia. J Pediatr 2012;160:88-92. [Crossref] [PubMed]
- Wurzel DF, Mackay IM, Marchant JM, et al. Adenovirus species C is associated with chronic suppurative lung diseases in children. Clin Infect Dis 2014;59:34-40. [Crossref] [PubMed]
- Wurzel DF, Marchant JM, Yerkovich ST, et al. Protracted bacterial bronchitis in children: natural history and risk factors for bronchiectasis. Chest 2016;150:1101-8. [Crossref] [PubMed]
- Amalakuhan B, Maselli DJ, Martinez-Garcia MA. Update in bronchiectasis 2014. Am J Respir Crit Care Med 2015;192:1155-61. [Crossref] [PubMed]
- Goyal V, Grimwood K, Marchant J, et al. Pediatric bronchiectasis: no longer an orphan disease. Pediatr Pulmonol 2016;51:450-69. [Crossref] [PubMed]
- Aliberti S, Masefield S, Polverino E, et al. EMBARC Study Group. Research priorities in bronchiectasis: a consensus statement from the EMBARC Clinical Research Collaboration. Eur Respir J 2016;48:632-47. [Crossref] [PubMed]
- Rogers DF. Pulmonary mucus: Pediatric perspective. Pediatr Pulmonol 2003;36:178-88. [Crossref] [PubMed]
- Jeffery PK. The development of large and small airways. Am J Respir Crit Care Med 1998;157:S174-80. [Crossref] [PubMed]
- Matsuba K, Thurlbeck WM. A morphometric study of bronchial and bronchiolar walls in children. Am Rev Respir Dis 1972;105:908-13. [PubMed]
- Chang AB, Byrnes CA, Everard ML. Diagnosing and preventing chronic suppurative lung disease (CSLD) and bronchiectasis. Paediatr Respir Rev 2011;12:97-103. [Crossref] [PubMed]
- Goyal V, Grimwood K, Marchant J, et al. Does failed chronic wet cough response to antibiotics predict bronchiectasis? Arch Dis Child 2014;99:522-5. [Crossref] [PubMed]
- Kumar A, Lodha R, Kumar P, et al. Non-cystic fibrosis bronchiectasis in children: clinical profile, etiology and outcome. Indian Pediatr 2015;52:35-7. [Crossref] [PubMed]
- Pasteur MC, Bilton D, Hill AT, et al. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010;65 Suppl 1:i1-58. [Crossref] [PubMed]
- King PT, Holdsworth SR, Farmer M, et al. Phenotypes of adult bronchiectasis: onset of productive cough in childhood and adulthood. COPD 2009;6:130-6. [Crossref] [PubMed]
- Vallejo AN. Immune remodeling: lessons from repertoire alterations during chronological aging and in immune-mediated disease. Trends Mol Med 2007;13:94-102. [Crossref] [PubMed]
- Carden KA, Boiselle PM, Waltz DA, et al. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest 2005;127:984-1005. [Crossref] [PubMed]
- Lee EY, Boiselle PM. Tracheobronchomalacia in infants and children: multidetector CT evaluation. Radiology 2009;252:7-22. [Crossref] [PubMed]
- Masters IB, Chang AB, Patterson L, et al. Series of laryngomalacia, tracheomalacia, and bronchomalacia disorders and their associations with other conditions in children. Pediatr Pulmonol 2002;34:189-95. [Crossref] [PubMed]
- Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology 2006;11:388-406. [Crossref] [PubMed]
- Masters IB, Zimmerman PV, Pandeya N, et al. Quantified tracheobronchomalacia disorders and their clinical profiles in children. Chest 2008;133:461-7. [Crossref] [PubMed]
- Majid A, Sosa AF, Ernst A, et al. Pulmonary function and flow-volume loop patterns in patients with tracheobronchomalacia. Respir Care 2013;58:1521-6. [Crossref] [PubMed]
- Su SC, Masters IB, Buntain H, et al. A comparison of virtual bronchoscopy versus flexible bronchoscopy in the diagnosis of tracheobronchomalacia in children. Pediatr Pulmonol 2017;52:480-6. [Crossref] [PubMed]
- Hallander HO, Gnarpe J, Gnarpe H, et al. Bordetella pertussis, Bordetella parapertussis, Mycoplasma pneumoniae, Chlamydia pneumoniae and persistent cough in children. Scand J Infect Dis 1999;31:281-6. [Crossref] [PubMed]
- Braman SS. Postinfectious cough: ACCP evidence-based clinical practice guidelines. Chest 2006;129:138S-146S. [Crossref] [PubMed]
- Atkinson SK, Sadofsky LR, Morice AH. How does rhinovirus cause the common cold cough? BMJ Open Respir Res 2016;3:e000118. [Crossref] [PubMed]
- Omar S, Clarke R, Abdullah H, et al. Respiratory virus infection up-regulates TRPV1, TRPA1 and ASICS3 receptors on airway cells. PLoS One 2017;12:e0171681. [Crossref] [PubMed]
- Wang K, Chalker V, Bermingham A, et al. Mycoplasma pneumoniae and respiratory virus infections in children with persistent cough in England. A retrospective analysis. Pediatr Infect Dis J 2011;30:1047-51. [Crossref] [PubMed]
- Shields MD, Thavagnanam S. The difficult coughing child: prolonged acute cough in children. Cough 2013;9:11. [Crossref] [PubMed]


