Induced pluripotent stem cells, form in vitro tissue engineering to in vivo allogeneic transplantation
Stem cell therapy brings a new hope and provides an alternative therapeutic strategy for treatment of human diseases in clinical therapy. Due to the stemness characteristics, stem cells are considered as a potential tool to understand and model many critical diseases such as Alzheimer’s (1), cardiovascular (2), cancer diseases (3) etc. Compared with multipotent embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), successfully generated from somatic cells by transfecting four transcription factors (Oct4, Sox2, Klf4, and c-Myc) in fibroblasts (4), could overcome the limitations of multipotent stem cells that commit to differentiate into only several lineage cells and the ethical concern of ESCs that need the oocytes and embryo destruction (5). Besides, iPSCs also possess abilities in generating other stem cells and a variety of immune cells for broad clinical requirements. Therefore, utilizing iPSCs in stem cell therapy has great attractions and provides tremendous applications in regenerative medicine (Figure 1).
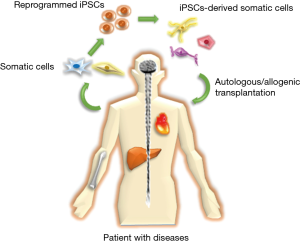
Recently, Shiba et al. first utilized the iPSCs to perform an allogeneic transplantation in primate heart for repairing the infarcted cardiac tissues (6), indicating that allogeneic transplantation of iPSC-derived cardiomyocytes (iPSC-CMs) did not require a large number of stem cells for heart regeneration. However, allogeneic transplantation could induce an immune response and cause graft rejection. Histocompatibility complex (MHC) plays an indispensable role in the immune response after transplantation and guest-host diseases. Based on the concept of MHC-matched transplantation, Shiba et al. directly injected iPSC-CMs from MHC haplotype (HT4) homozygous cynomolgus monkey (Macaca fascicularis), which MHC structure is equal to that of human, into the HT4 heterozygous monkeys with myocardial infarction. After 12 weeks of transplantation, the grafted iPSC-CMs still survived and improved the cardiac contractile function with no evidence of immune rejection. These results demonstrated that the feasibility of allogeneic transplantation by iPSCs from primates for repairing clinical human diseases.
The iPSC-CM engineered cardiac tissue present great opportunities for regenerative medicine, drug screening, and disease modeling. However, recent studies found that hiPSC-CMs could not accurately replicate the native morphology and function of CMs in adult heart due to the complexity of structure and function of the heart (7,8). To this end, researchers have applied various approaches, including electric stimulus, cyclic stretch and chemical molecules, to replicate the in vivo myocardium microenvironment to promote the iPSC-CM maturation (9-11). Shiba et al. also found that the expression of cTnT was lower in the allogeneic iPSC-CMs than in the adult hearts, indicating the immaturity of iPSC-CMs in vitro (6). Therefore, cells were treated with pro-survival cocktail before transplantation. The in vivo grafted iPSC-CMs were electrically integrated with the host myocardium. In addition, the iPSCs could also differentiate into other cells to achieve a wide range of applications, such as ophthalmic, neural and bone tissues.
Retina is a one of important tissues in eyes because it contains a simple epithelial monolayer, which involves retinol cycling and nutrient transport of photoreceptors. Many retinal diseases such as age-related macular degeneration (AMD), retinitis pigmentosa (RP), and Stargardt’s disease (STGD) are related to the degeneration of photoreceptor cells leading to vision loss or blindness. However, there is no efficient curative treatment for these diseases. Recently, Zhong et al. successfully induced human iPSCs to differentiate, recapitulate the main steps of retinal development, and form a three dimensional (3D)-retinal cups with mature photoreceptors (12). Furthermore, Cyranoski showed a case that the first person with AMD whose vision was recovered by implanting a retinal pigment epithelium cell sheet generated from autologous iPSCs of a patient into an eye (13). Additionally, several biomaterials such as chitosan, collagen, and gelatin etc. are widely used in ocular tissue engineering for corneal regeneration (14). These biomaterials, especially collagen and gelatin, are indicated having great cell viability and biocompatibility as a carrier to encapsulate limbal epithelial cells (LEC), corneal endothelial cells, and stromal cells (15,16) or as an ocular drug delivery carrier (17). Moreover, Collagen and fibrin hydrogels shows strong abilities for inducing corneal and LEC differentiation of mesenchymal stem cells (MSCs) (18,19) to re-epithelialized corneal tissues of patients with vision loss (19,20). It implied that combination of stem cells and biomaterials is a potential therapeutic in ocular tissue regeneration. Chiou et al. combined keratocyte-reprogrammed iPSCs and thermosensitive chitosan-based hydrogel to repair corneal wound healing (21). Combination of iPSCs and biomaterials as a stem cell niche provides the proliferation of endogenous limbal stem cells, increases the epithelial cellular growth, and recovers the thickness of corneal epithelium in damaged corneas. It shows the potential applications of iPSCs in ocular tissue engineering for clinical regeneration.
Neurological diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and ischemic stroke etc. are major global health burdens in the world (22). The common mechanisms resulting in these diseases are the accumulation of misfolded aggregated proteins or lack of the blood flow yielding neuron death (23). However, there are no significant radical methods for neuronal regeneration. iPSCs via regulation of medium components have already showed their abilities, generating neural progenitor cells, differentiating into functionally specialized neurons (brain/motor neurons) and glia cells (astrocyte) (22). Therefore, modeling neurological diseases by iPSCs is a novel concept in clinical medicine. Compared with transplantation of fetal neural stem/precursor cells (NSPCs) or fetal neural tissues, the use of primary fetal neural tissues or NSPCs lacks scalable ability, and has the recurrence of dyskinesia and ethical issues. Contrarily, the above issues are less mentioned in using iPSCs as a therapeutic tool (24,25). For the clinical potential applications, transplanting autologous/allogeneic stroma-reprogrammed mouse iPSCs in rat brains showed the midbrain-like dopamine neuron from transplanted iPSCs improving the rats with PD (26,27). Additionally, to control iPSCs proliferating and differentiating into specific subtype neurons by biomaterials are widely investigated recently. For example, by the thickness of electrospun fibers as 3D microenvironments regulating cell-cell contact interactions supports the reprogrammed neuron from iPSCs with high ectopic expression of NeuroD1 (iNs) (28). Furthermore, the in vivo results also showed the iNs on electrospun structure were with the high survival rate and the synaptophysin-expressed neurite termianls indicating synaptic connection with host brain tissues (28). Besides, Wang et al. fabricated a nanofibrous tubular conduit as a bridge to induce iPSCs differentiating into Schwann cells, connecting transected sciatic nerves, and accelerating regeneration of sciatic nerves (29), these show the feasibilities of combining iPSCs and biomaterials to regenerate the diseases of central and peripheral nerve system in clinic applications.
Bone defects such as trauma or orthopedic defects make disability in patients. Allogenic or autogenic bone graft transplantation are gold standards for major treatments. However, weak osteoinduction, donor availability, and cost etc. are the limitations in autogenic/allogenic transplantation (30). In past decade, utilizing engineered bone instead of autogenic/allogeneic bone is widely used in clinical (31). But, it requires other cell sources to create vascular and nerve compartments. Therefore, combination of stem cells and biomaterials has gradually developed as an alternative strategy for complex bon reconstruction (32). MSCs isolated from adult tissues are the most commonly used stem cells in bone regeneration. However, following aging, the functionality in regeneration and self-renewal ability of MSCs are impaired (33). Compared with MSCs, Sheyn et al. reported that BMP6-overexpressing MSC-generated from iPSCs (iMSCs) by short-term exposing transforming growth factor-β (TGF-β) could overcome the challenges, possessing self-renewal without tumorigenic ability, acquiring more differentiated cell type, and regenerating bone defects (34). Additionally, de Peppo et al. also confirmed that different human iPSCs lines (hiPSCs) (11c, 1013A, and BC1) on the engineered bone constructs were strongly induced toward the expressions of osteoblast differentiations and bone formation (STAT3 and TGFB3 genes) (35). Recently, various biomaterials are developed for the regeneration of bone defects (36). Due to the stiffness of scaffolds playing a role in ontogenesis differentiation of stem cells (37), tunable biomaterials such as polyethylene glycol (PEG)- gelatin-, or poly (vinyl alcohol) (PVA)- based hydrogels (38-40) etc. are gotten attention for bone regenerative engineering. Moreover, most shapes of bone defects are irregular so that implanted scaffolds with regular shapes have low regenerative efficiency in juried bones. To overcome this limitation, Faulkner-Jones et al. first showed a iPSC-laden ink for bioprinting (41). Therefore, combination of iPSC-laden inks, current 3D bioprinting technologies, and computed tomography scan brings a new concept for applications of iPSCs in bone tissue engineering.
The iPSC technology brings a new hope as a probably therapeutic tool for cell therapies. However, the safety and effectiveness of iPSC transplantation in disease remodeling should be evaluated carefully. Some challenges including specific differentiated protocols or immune-response exist to interrupt the transplantation of iPSCs becoming a reality in clinical medicine so that iPSCs therapy is still at the preliminary stage. Recently, many studies showed using iPSC-derived specific lineage progenitor cells to treat human disorders (42-46). It shows that using customized reprogrammed iPSC-derived progenitor cells will provide a strategy to enhance the regenerative efficiency of specific human diseases. The safety issues of iPSCs such as tumorigenicity are gotten attention because of using retrovirus or lentivirus as the vectors. But following the new reprogrammed protocols without exogenous DNA integration (47,48) or with only small molecule compounds (49), the risks of tumor formation could be decreased. Besides, El Khatib’s and Shiba’s groups successfully utilized autologous/allogeneic iPSCs to treat islet and heart diseases without any tumorigenicity (6,50), these results increases the applicability of iPSCs in clinical medicine. Additionally, despite many disease remodeling by using iPSCs, regeneration of complex 3D tissues or organs is a major challenge in regenerative medicine. In past decade, combination of stem cells and scaffolds for tissue/organ regeneration is spring up in tissue engineering field. Notably, to overcome the fabricated limitations of complex tissues/organs, 3D and 4D bioprinting technologies create a new vision for fabrication of complex structures (51). By the bioprinting technologies, the stem cell-laden structures could mimic the complex environments in body with the dynamic responses where is close to the native human tissues/organs. Therefore, combining autologous/allogeneic iPSCs, biomaterials, and bioprinting technologies to regenerate human diseases could be expected as a new trend in the next-generation therapeutic methods in clinical medicine.
Acknowledgements
The paper was supported by Taiwan Bio-Development Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kang JM, Yeon BK, Cho SJ, et al. Stem Cell Therapy for Alzheimer's Disease: A Review of Recent Clinical Trials. J Alzheimers Dis 2016;54:879-89. [Crossref] [PubMed]
- Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature 2008;451:937-42. [Crossref] [PubMed]
- Mohammadi M, Jaafari MR, Mirzaei HR, et al. Mesenchymal stem cell: a new horizon in cancer gene therapy. Cancer Gene Ther 2016;23:285-6. [Crossref] [PubMed]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76. [Crossref] [PubMed]
- Liang G, Zhang Y. Embryonic stem cell and induced pluripotent stem cell: an epigenetic perspective. Cell Res 2013;23:49-69. [Crossref] [PubMed]
- Shiba Y, Gomibuchi T, Seto T, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016;538:388-91. [Crossref] [PubMed]
- Sarić T, Halbach M, Khalil M, et al. Induced pluripotent stem cells as cardiac arrhythmic in vitro models and the impact for drug discovery. Expert Opin Drug Discov 2014;9:55-76. [Crossref] [PubMed]
- Lee P, Klos M, Bollensdorff C, et al. Simultaneous voltage and calcium mapping of genetically purified human induced pluripotent stem cell-derived cardiac myocyte monolayers. Circ Res 2012;110:1556-63. [Crossref] [PubMed]
- Zhu R, Blazeski A, Poon E, et al. Physical developmental cues for the maturation of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther 2014;5:117. [Crossref] [PubMed]
- Cao H, Kang BJ, Lee CA, et al. Electrical and Mechanical Strategies to Enable Cardiac Repair and Regeneration. IEEE Rev Biomed Eng 2015;8:114-24. [Crossref] [PubMed]
- Ruan JL, Tulloch NL, Razumova MV, et al. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 2016;134:1557-67. [Crossref] [PubMed]
- Zhong X, Gutierrez C, Xue T, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun 2014;5:4047. [Crossref] [PubMed]
- Reardon S, Cyranoski D. Japan stem-cell trial stirs envy. Nature 2014;513:287-8. [Crossref] [PubMed]
- Wright B, Mi S, Connon CJ. Towards the use of hydrogels in the treatment of limbal stem cell deficiency. Drug Discov Today 2013;18:79-86. [Crossref] [PubMed]
- Fagerholm P, Lagali NS, Merrett K, et al. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med 2010;2:46ra61. [Crossref] [PubMed]
- Watanabe R, Hayashi R, Kimura Y, et al. A novel gelatin hydrogel carrier sheet for corneal endothelial transplantation. Tissue Eng Part A 2011;17:2213-9. [Crossref] [PubMed]
- Hori K, Sotozono C, Hamuro J, et al. Controlled-release of epidermal growth factor from cationized gelatin hydrogel enhances corneal epithelial wound healing. J Control Release 2007;118:169-76. [Crossref] [PubMed]
- Ma Y, Xu Y, Xiao Z, et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells 2006;24:315-21. [Crossref] [PubMed]
- Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 2010;363:147-55. [Crossref] [PubMed]
- Fagerholm P, Lagali NS, Ong JA, et al. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials 2014;35:2420-7. [Crossref] [PubMed]
- Chien Y, Liao YW, Liu DM, et al. Corneal repair by human corneal keratocyte-reprogrammed iPSCs and amphiphatic carboxymethyl-hexanoyl chitosan hydrogel. Biomaterials 2012;33:8003-16. [Crossref] [PubMed]
- Russo FB, Cugola FR, Fernandes IR, et al. Induced pluripotent stem cells for modeling neurological disorders. World J Transplant 2015;5:209-21. [Crossref] [PubMed]
- Avila J. Common mechanisms in neurodegeneration. Nat Med 2010;16:1372. [Crossref] [PubMed]
- D'Aiuto L, Zhi Y, Kumar Das D, et al. Large-scale generation of human iPSC-derived neural stem cells/early neural progenitor cells and their neuronal differentiation. Organogenesis 2014;10:365-77. [Crossref] [PubMed]
- Trounson A, DeWitt ND. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol 2016;17:194-200. [Crossref] [PubMed]
- Wernig M, Zhao JP, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A 2008;105:5856-61. [Crossref] [PubMed]
- Sundberg M, Bogetofte H, Lawson T, et al. Improved cell therapy protocols for Parkinson's disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem Cells 2013;31:1548-62. [Crossref] [PubMed]
- Carlson AL, Bennett NK, Francis NL, et al. Generation and transplantation of reprogrammed human neurons in the brain using 3D microtopographic scaffolds. Nat Commun 2016;7:10862. [Crossref] [PubMed]
- Wang A, Tang Z, Park IH, et al. Induced pluripotent stem cells for neural tissue engineering. Biomaterials 2011;32:5023-32. [Crossref] [PubMed]
- Rogers GF, Greene AK. Autogenous bone graft: basic science and clinical implications. J Craniofac Surg 2012;23:323-7. [Crossref] [PubMed]
- Fröhlich M, Grayson WL, Wan LQ, et al. Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance. Curr Stem Cell Res Ther 2008;3:254-64. [Crossref] [PubMed]
- Meijer GJ, de Bruijn JD, Koole R, et al. Cell-based bone tissue engineering. PLoS Med 2007;4:e9. [Crossref] [PubMed]
- Duscher D, Rennert RC, Januszyk M, et al. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci Rep 2014;4:7144. [Crossref] [PubMed]
- Sheyn D, Ben-David S, Shapiro G, et al. Human Induced Pluripotent Stem Cells Differentiate Into Functional Mesenchymal Stem Cells and Repair Bone Defects. Stem Cells Transl Med 2016;5:1447-60. [Crossref] [PubMed]
- de Peppo GM, Marcos-Campos I, Kahler DJ, et al. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc Natl Acad Sci U S A 2013;110:8680-5. [Crossref] [PubMed]
- Yu X, Tang X, Gohil SV, et al. Biomaterials for Bone Regenerative Engineering. Adv Healthc Mater 2015;4:1268-85. [Crossref] [PubMed]
- Zhao W, Li X, Liu X, et al. Effects of substrate stiffness on adipogenic and osteogenic differentiation of human mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl 2014;40:316-23. [Crossref] [PubMed]
- Caiazzo M, Okawa Y, Ranga A, et al. Defined three-dimensional microenvironments boost induction of pluripotency. Nat Mater 2016;15:344-52. [Crossref] [PubMed]
- Helminger M, Wu B, Kollmann T, et al. Synthesis and Characterization of Gelatin-Based Magnetic Hydrogels. Adv Funct Mater 2014;24:3187-96. [Crossref] [PubMed]
- Kim TH, An DB, Oh SH, et al. Creating stiffness gradient polyvinyl alcohol hydrogel using a simple gradual freezing-thawing method to investigate stem cell differentiation behaviors. Biomaterials 2015;40:51-60. [Crossref] [PubMed]
- Faulkner-Jones A, Fyfe C, Cornelissen DJ, et al. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015;7:044102. [Crossref] [PubMed]
- Schwartz RE, Trehan K, Andrus L, et al. Modeling hepatitis C virus infection using human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109:2544-8. [Crossref] [PubMed]
- Trounson A, Shepard KA, DeWitt ND. Human disease modeling with induced pluripotent stem cells. Curr Opin Genet Dev 2012;22:509-16. [Crossref] [PubMed]
- Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol 2011;13:497-505. [Crossref] [PubMed]
- Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 2007;318:1920-3. [Crossref] [PubMed]
- Lu TY, Lin B, Kim J, et al. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun 2013;4:2307. [Crossref] [PubMed]
- Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009;4:472-6. [Crossref] [PubMed]
- Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 2009;4:381-4. [Crossref] [PubMed]
- Hou P, Li Y, Zhang X, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 2013;341:651-4. [Crossref] [PubMed]
- El Khatib MM, Ohmine S, Jacobus EJ, et al. Tumor-Free Transplantation of Patient-Derived Induced Pluripotent Stem Cell Progeny for Customized Islet Regeneration. Stem Cells Transl Med 2016;5:694-702. [Crossref] [PubMed]
- Li YC, Zhang YS, Akpek A, et al. 4D bioprinting: the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016;9:012001. [Crossref] [PubMed]


