The future of hybrid ablation: an emerging need for an anticoagulation protocol for thoracoscopic ablation
With great interest, we read the article by de Asmundis et al. regarding midterm clinical outcomes of concomitant thoracoscopic epicardial and catheter endocardial ablation for persistent and long-standing persistent atrial fibrillation (AF) (1). Several points in the article stood out, of which we will mention three. First, the very good efficacy of the procedure in a population of patients with persistent and long-standing persistent AF, second, the relatively high number of complications associated with thoracoscopic procedure, and third, the absence of a complete description of anticoagulation used during their thoracoscopic ablation procedure (which may seem like a small thing, however, in the context of other similar studies, is of great importance).
Thoracoscopic ablation—reported efficacy
The reported efficacy in the published study by de Asmundis was that 67.2% of patients were in sinus rhythm and no longer needed antiarrhythmic drugs (AAD) 2 years after ablation (1). Keeping in mind that all patients had persistent and long-standing persistent AF, the efficacy was very good. Similar efficacy of hybrid ablations, in the midterm, has been described by others. Pison et al. reported a success rate of 87% AF-freedom in a series of 78 patients after a median follow-up of 24 months (2). As with de Asmundis, the thoracoscopic procedure was done using a bilateral bipolar AtriCure clamp + linear pen device. However, Pison et al. reported a mixed population of paroxysmal and non-paroxysmal AF patients; in a subgroup of non-paroxysmal AF (mainly persistent), the efficacy was 82% at 2 years. On et al. reported on 78 (paroxysmal and non-paroxysmal) patients undergoing hybrid ablation (bipolar clamp + pen surgical device in two-staged design) with AF freedom of 92.6% at 2 years (3). Bulava et al. reported the results of two-staged, hybrid ablation of 50 patients, all with non-paroxysmal AF (4). In contrast to the de Asmundis study, the catheter ablation stage was done 3 months later after the thoracoscopic stage. With a mean follow-up of 12 months, AF freedom was seen in 94% (but on AADs and ablations) (4). Thus, the results of de Asmundis were fully comparable for that series.
According to a recent meta-analysis of 12 observational studies of patients after hybrid ablation of AF, in studies using bipolar ablation devices, the success rate (with AADs) was 71% after a mean of 26 months (both paroxysmal and nonparoxysmal patients) (5). Keeping in mind that the majority of patients were in persistent or long standing persistent AF, the efficacy seems to be very good.
The exact comparison of efficacy between the mentioned studies is difficult because there were significant differences between the surgical approaches. All hybrid procedures were performed off-pump, mini-invasively, using the thoracoscopic approach. However, the ablation procedure itself varied considerably. The “common denominator” for all procedures was the creation of a box lesion of the posterior left atrium (LA) (i.e., en bloc encircling all four pulmonary veins and posterior aspect of the LA). A box lesion represents the “core” of the Cox-Maze III/IV lesion set of the LA, and was used by all cardiac surgeons in the mentioned studies. However, the number and location of additional ablation lines created during thoracoscopic ablation varied significantly. Some authors prefer a more complex ablation of the LA, which makes, in the end (after subsequent catheter ablation) the LA ablation set very close to the left atrial part of the Cox-Maze III/IV procedure. These additional lines included the mitral isthmus line, trigone line, occlusion of the appendage of the left atrium (LAA) with a line from LAA to the left superior pulmonary vein, or dissection of the ligament of Marshal. Other authors preferred box lesions in the LA only without any additional lines. Differences in right sided lesions also varied substantially. Some authors created complex right-sided lesions [i.e., superior caval vein (sup. vena cava) isolation, an intercaval line, and a cavotricuspid line during catheter ablation]; other authors did not touch right atrium at all. The ablation used by de Asmundis was more complex on the right side (in 53% patients with dilated atria, an intercaval line and isolation of the superior caval vein was performed), on the other hand, the thoracoscopic ablation was more conservative on the left side (epicardial mitral isthmus ablation in only 6% of patients; with a standard box lesion in the rest). The heterogeneity between studies prevents us from directly comparing the results. No randomized study comparing these different approaches is underway. However, since the original goal of the hybrid ablation was to perform very complex ablations (Cox-Maze III/IV-like) with minimal invasiveness (thoracoscopically, off-pump), more complex lesion sets (on both sides) could have an advantage.
Safety of hybrid ablation
Safety, not efficacy, seems to be the Achilles heel of hybrid (thoracoscopic) ablations. Two patients (3%) experienced LA perforation requiring urgent sternotomy (1). The authors themselves recognized these complications as life threatening. Fortunately, the outcome for both patients was good. Similarly, Boersma et al. reported one sternotomy in a series of 61 (1.6%) patients (6), and two (3.9%) conversions to sternotomy due to bleeding from the left pulmonary artery was reported also by Bulava (4). Moreover, de Asmundis reported that 11 (17%) other patients developed complications within the 30-day postoperative period. Fortunately, the majority of them, such as pneumonia or pneumothorax, were short-term with no long-term sequela. Similar results, i.e., higher efficacy and worse safety, were reported also in the randomized study by Boersma (6).
Stroke risk and thoracoscopic ablation
No strokes were reported by de Asmundis; however, the rate of strokes in other reports of hybrid ablations (specifically during the thoracoscopic part of the procedure) are relatively high, and unfortunately, and unlike a pneumothorax or pneumonia, the consequences of stroke are long-term. Recently, Romanov et al. reported a study comparing two approaches to thoracoscopic ablation for AF: thoracoscopic box lesion vs. box lesion plus occlusion of the LAA. For the whole series, they reported 6/176 (3.4%) perioperative strokes or TIAs in a population of “low-risk” AF patients (7). One stroke in 61 patients (1.6%) was reported by Boersma and one stroke in 30 patients (3.3%) was reported in one of our recent publications (8). On et al. described peri-operative strokes in 2.5% of patients (2 out of 79) during thoracoscopic ablation. Probst reported strokes/TIAs in 5.1% of patients (9), Edgerton reported peri-operative strokes in 4.2% of patients (10), and Compier reported 4% of strokes being present shorty after thoracoscopic ablation (11). Although, in the only direct randomized comparison, Boersma (6) reported that the incidence of stroke was the same in catheter endocardial and thoracoscopic ablation groups (i.e., 1.6% for each group). The occurrence of strokes in other observational studies of hybrid ablations seems to point to higher thoracoscopic-stage rates compared to catheter-stage rates.
Several factors could lead to higher stroke rates during thoracoscopic ablations. Although the ablation is done epicardially, the transmurality of the lesion might lead to damage on the endocardial surface and a greater risk of thrombus development in the LA. Furthermore, since the majority of patients suffer from persistent or long-standing persistent AF (e.g., in the de Asmundis report, 92% of patients were in AF at the beginning of the surgery) (1), and thus most of them are cardioverted to sinus rhythm during ablation (either by the ablation itself or by direct current cardioversion), the resulting left atrial stunning (which is recognized as pro-thrombus) could contribute to thrombus formation (12). Lastly, in most studies, no anticoagulation was given during the thoracoscopic ablation (or at least no heparin was reported given).
In the studies that used a one-stage design, i.e., studies in which the thoracoscopic ablation was immediately followed by EP and catheter ablation, heparin administration during the endocardial-phase was described in the methods section of these manuscript very preciously. Heparin was administered as recommended for endocardial ablation with a target activated clotting time (ACT) of more than 300 or 350 s. The number of reported strokes for hybrid ablations using the one-stage design is also low (Table 1). However, even in those studies, information regarding heparin administration during the thoracoscopic-phase of the procedure was missing (probably, no heparin was given).
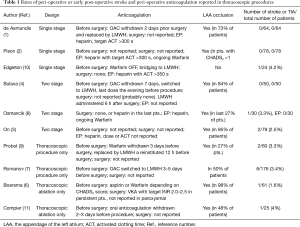
Full table
Because the risk of stroke seems to be higher during thoracoscopic ablations, one would expect very precise reporting of anticoagulation used peri-operatively to address this issue. Unfortunately, the opposite seems to be true: very few studies fully report the anticoagulation management used during surgery and immediately afterwards (Table 1). This is in huge contrast to catheter ablation papers, in which the anticoagulation protocol is described in detail, e.g., exact international normalized ration on the day of ablation, heparin dose given before and after trans-septal puncture, etc. In articles describing thoracoscopic ablations, typically the only anticoagulation information given is with regard to withdrawal of Warfarin or NOACs before the ablation. The absence of a description of the anticoagulation protocol, in procedures with a high risk of stroke, is hard to justify.
Future of hybrid ablation—a need for standard anticoagulation protocol
The efficacy of hybrid ablation seems to be very promising. The thoracoscopic procedure could be more standardized to make the results more comparable, and more complex ablation procedures (similar to Cox-Maze III/IV lesion set) should be preferred. However, the major limitation of the hybrid or thoracoscopic approach seems to be safety. In contrast to EP studies, which are easier to perform in many centers, the number of patients enrolled in thoracoscopic or hybrid studies has been substantially lower. Thoracoscopic or hybrid ablations are still considered to be the tertiary treatment of AF and only for selected patients. The procedure is technically challenging, and is not routinely offered by the majority of cardiac surgery departments. Typically, only patients with persistent or long-standing persistent AF, with previous but unsuccessful catheter ablations, or patients with dilated atria are considered for hybrid or thoracoscopic ablations. Moreover, the exclusion criteria for thoracoscopic ablation are also more restrictive, respecting lung function or other comorbidities, which could increase the risk of surgery. Therefore, even in the largest, high-volume centers, we cannot expect a study enrolling hundreds of patients, like we see with catheter ablation studies, and currently no multicenter randomized studies covering this topic are underway.
Stroke present the most dangerous complication of ablation, in contrast to other complications (such as pneumothorax, bleeding etc. which consequences disappear soon after surgery), the consequences of stroke are present for long term. As it is mentioned, the rate of strokes seems to be higher during thoracoscopic ablation. It is warranted: in such situation, the anticoagulation management should be reported very preciously in detail in all papers to solve this issue and to establish the best anticoagulation strategy. Nowadays, no recommendations for perioperative anticoagulation during thoracoscopic procedure are present either in the recent ESC guidelines (13), or in the recently published guidelines of the Society of Thoracic Surgeons (14).
For now, at least, any conclusions regarding the efficacy and safety have to be drawn from meta-analyses of reported (mainly observational) trials, and this can only happen if the trials contain the necessary data. To this end, full descriptions of anticoagulation protocols are essential. We understand that anticoagulation during thoracoscopic ablation is difficult task, on one hand, avoidance of thrombi formation is critical, on the other hand, avoidance of excessive bleeding is just as critical. We feel it is crucial that all future publications on thoracoscopic ablation include a full description of the exact anticoagulation protocol used, just as it is fully described in catheter ablation studies. A discussion of the best anticoagulation strategy during thoracoscopic ablation should be started. Hopefully, in the near future, a standardized peri- and post-operative anticoagulation strategy can be established, just as it was established for catheter ablations.
Acknowledgements
Funding: The paper was supported by the Ministry of Health of the Czech Republic, research grant Nr. AZV 16-32478A.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- de Asmundis C, Chierchia GB, Mugnai G, et al. Midterm clinical outcomes of concomitant thoracoscopic epicardial and transcatheter endocardial ablation for persistent and long-standing persistent atrial fibrillation: a single-centre experience. Europace 2017;19:58-65. [PubMed]
- Pison L, Gelsomino S, Lucà F, et al. Effectiveness and safety of simultaneous hybrid thoracoscopic and endocardial catheter ablation of lone atrial fibrillation. Ann Cardiothorac Surg 2014;3:38-44. [PubMed]
- On YK, Park KM, Jeong DS, et al. Electrophysiologic Results After Thoracoscopic Ablation for Chronic Atrial Fibrillation. Ann Thorac Surg 2015;100:1595-602; discussion 1602-3. [Crossref] [PubMed]
- Bulava A, Mokracek A, Hanis J, et al. Sequential hybrid procedure for persistent atrial fibrillation. J Am Heart Assoc 2015;4:e001754. [Crossref] [PubMed]
- Vroomen M, Pison L. Hybrid ablation for atrial fibrillation: a systematic review. J Interv Card Electrophysiol 2016;47:265-74. [Crossref] [PubMed]
- Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23-30. [Crossref] [PubMed]
- Romanov A, Pokushalov E, Elesin D, et al. Effect of left atrial appendage excision on procedure outcome in patients with persistent atrial fibrillation undergoing surgical ablation. Heart Rhythm 2016;13:1803-9. [Crossref] [PubMed]
- Osmancik P, Budera P, Zdarska J, et al. Electrophysiological findings after surgical thoracoscopic atrial fibrillation ablation. Heart Rhythm 2016;13:1246-52. [Crossref] [PubMed]
- Probst J, Jidéus L, Blomström P, et al. Thoracoscopic epicardial left atrial ablation in symptomatic patients with atrial fibrillation. Europace 2016;18:1538-44. [Crossref] [PubMed]
- Edgerton Z, Perini AP, Horton R, et al. Hybrid Procedure (Endo/Epicardial) versus Standard Manual Ablation in Patients Undergoing Ablation of Longstanding Persistent Atrial Fibrillation: Results from a Single Center. J Cardiovasc Electrophysiol 2016;27:524-30. [Crossref] [PubMed]
- Compier MG, Braun J, Tjon A, et al. Outcome of stand-alone thoracoscopic epicardial left atrial posterior box isolation with bipolar radiofrequency energy for longstanding persistent atrial fibrillation. Neth Heart J 2016;24:143-51. [Crossref] [PubMed]
- Khan IA. Atrial stunning: basics and clinical considerations. Int J Cardiol 2003;92:113-28. [Crossref] [PubMed]
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893-962. [Crossref] [PubMed]
- Badhwar V, Rankin JS, Damiano RJ Jr, et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann Thorac Surg 2017;103:329-41. [Crossref] [PubMed]


